1. A reaction is \[A+B\rightleftharpoons C+D\] . Initially we start with equal concentrations of A and B. At equilibrium we find that the moles of C is two times of A. What is the equilibrium constant of the reaction?
a) \[\frac{1}{4}\]
b) \[\frac{1}{2}\]
c) 4
d) 2
Explanation:
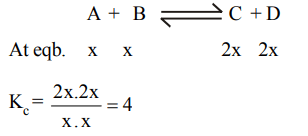
2. In which of the following, the forward reaction is favoured by use of high pressure?
a) \[H_{2}+I_{2}\rightleftharpoons2HI\]
b) \[N_{2}+O_{2}\rightleftharpoons2NO\]
c) \[2NH_{3}\rightleftharpoons N_{2}+3H_{2}\]
d) \[2SO_{2}+O_{2}\rightleftharpoons2SO_{3}\]
Explanation: \[2SO_{2}+O_{2}\rightleftharpoons2SO_{3}\]
3. In a reaction \[A + B\rightleftharpoons C+D\] , the initial concentrations, of A and B were 0.9 mol. dm-3 each. At equilibrium the
concentration of D was found to be 0.6 mol \[dm^{-3}\]. What is the value of equilibrium constant for the reaction
a) 8
b) 4
c) 9
d) 3
Explanation:

4. On the basis of Le-Chatelier's principle, predict which of the following conditions would be unfavourable for the formation of \[SO_{3}\]? Given that
\[2SO_{2}+O_{2}\rightleftharpoons 2SO_{3}\] ; \[\triangle H =-42 kcal\]
a) Low pressure and low temperature
b) High pressure and low temperature
c) High temperature and low pressure
d) High concentration of \[SO_{2}\]
Explanation: Le Chatelier's principle since reaction is exothermic hence low temperature will favour forward reaction also volume is decreased by applying high pressure.
5. If the equilibrium constant for the reaction \[2AB\rightleftharpoons A_{2} +B_{2}\] is 49, what is the value of equilibrium constant for
\[AB\rightleftharpoons\frac{1}{2} A_{2} +\frac{1}{2}B_{2}\]
a) 49
b) 2401
c) 7
d) 0.02
Explanation: Reaction (II) is \[\frac{1}{2}\] of (I)
\[K = \sqrt{K} = \sqrt{49} = 7\]
6. The reaction, \[SO_{2}+Cl_{2}\rightarrow SO_{2}Cl_{2}\] is exothermic and reversible. A mixture of\[SO_{2}\left(g\right), Cl_{2}\left(g\right) and SO_{2}Cl_{2}\left(l\right)\] is at equilibrium in a closed container. Now a certain quantity of
extra \[SO_{2}\] is introduced into the container, the volume
remaining the same. Which of the following is/are true?
a) The pressure inside the container will not change
b) The temperature will not change
c) The temperature will increase
d) The temperature will decrease
Explanation: By addition of SO2 equilibrium will shift to RHS which is exothermic. Hence temperature will increase.
7. The rate of forward reaction is two times that of the reverse reaction at a given temperature and identical concentration. \[K_{equilibrium}\] is
a) 0.5
b) 1.5
c) 2.5
d) 2.0
Explanation:
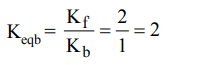
8. 1 mole of hydrogen and 2 moles of iodine are taken initially in a 2 litre vessel. The number of moles of hydrogen at equilibrium is 0.2. Then the number of moles of iodine and hydrogen iodide at equilibrium are
a) 1.2, 1.6
b) 1.8, 1.0
c) 0.4, 2.4
d) 0.8, 2.0
Explanation:
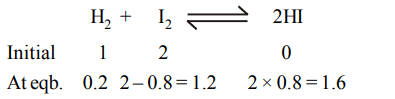
9. For the reaction \[PCl_{5}\left(g\right)\rightleftharpoons PCl_{3}\left(g\right)+CI_{2}\left(g\right)\] the forward reaction at constant temperature is favoured by
a) introducing an inert gas at constant volume
b) introducing \[PCl_{3}\left(g\right)\] at constant volume
c) introducing \[PCl_{5}\left(g\right)\] at constant volume
d) introducing \[Cl_{2}\left(g\right)\] at constant volume
Explanation: introducing \[PCl_{5}\left(g\right)\] at constant volume
10. For the chemical reaction :
\[3X\left(g\right)+Y\left(g\right)\rightleftharpoons X_{3}Y \left(g\right)\]
the amount of \[X_{3}Y\] at equilibrium is affected by
a) temperature and pressure
b) pressure only
c) temperature only
d) temperature, pressure and catalyst
Explanation: Temperature and pressure (Le Chatelier's principle) \[\triangle\]n \[\neq\] 0 .