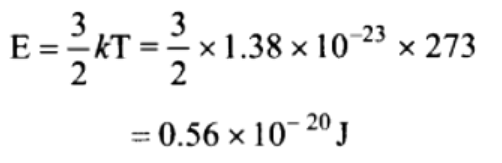1. The mean kinetic energy of a gas at 300 K is 100 J. The mean energy of the gas at 450 K is equal to
a) 100 J
b) 3000 J
c) 450 J
d) 150 J
Explanation:

2. The capacity of a vessel is 3 litres. It contains 6 gm oxygen, 8 gm nitrogen and 5 gm \[CO_{2}\] mixture at 27°C. If R = 8.31 \[J/mole\times kelvin\] , then the
pressure in the vessel in \[N\diagup m^{2}\] will be (approx.)
a) \[5\times10^{5}\]
b) \[5\times10^{4}\]
c) \[10^{6}\]
d) \[10^{5}\]
Explanation:
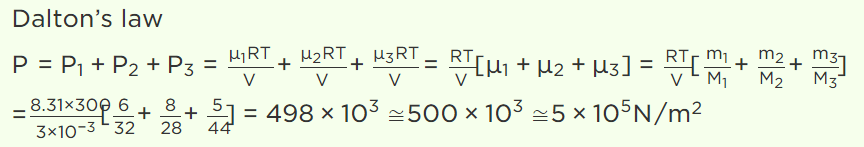
3. In the absence of intermolecular force of attraction, the observed pressure P will be
a) P
b) < P
c) > P
d) Zero
Explanation: In the absence of intermolecular force of attraction, the observed pressure P will be > P
4. Two ideal gases at absolute temperature \[T_{1}\] and \[T_{2} \] are mixed. There is no loss of energy. The masses of the molecules are \[m_{1}\] and \[m_{2} \] and the number of
molecules in the gases are \[n_{1}\] and \[n_{2} \] respectively. The temperature of mixture will be
a) \[\frac{T_{1} + T_{2} }{2}energy\]
b) \[\frac{T_{1} + T_{2} }{n_{1} n_{2}}\]
c) \[\frac{n_{1}T_{1} +n_{2} T_{2} }{n_{1}+ n_{2}}\]
d) \[\left(T_{1} + T_{2}\right)\]
Explanation: \[\frac{n_{1}T_{1} +n_{2} T_{2} }{n_{1}+ n_{2}}\]
5. The molecules of an ideal gas at a certain temperature have
a) Only potential energy
b) Only kinetic energy
c) Potential and kinetic energy both
d) None of the above
Explanation: The molecules of an ideal gas at a certain temperature have only kinetic energy
6. Mean kinetic energy per degree of freedom of gas molecules is
a) \[\frac{3}{2}kT\]
b) kT
c) \[\frac{1}{2}kT\]
d) \[\frac{3}{2}RT\]
Explanation: Mean kinetic energy per degree of freedom of gas molecules is \[\frac{1}{2}kT\]
7. The temperature at which the average translational kinetic energy of a molecule is equal to the energy gained by an electron in
accelerating from rest through a potential difference of 1 volt is
a) \[4.6\times 10^{3}K\]
b) \[11.6\times 10^{3}K\]
c) \[23.2\times 10^{3}K\]
d) \[7.7\times 10^{3}K\]
Explanation:
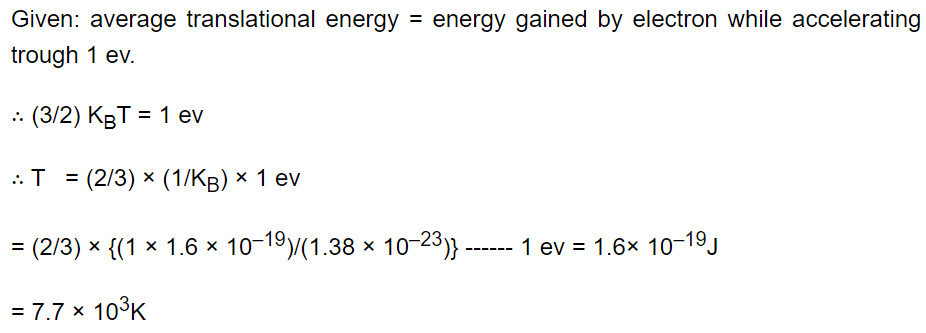
8. The kinetic energy of one gm-mole of a gas at normal temperature and pressure is (R = 8.31
J/Mole-K)
a) \[0.56\times 10^{4}J\]
b) \[1.3\times 10^{2}J\]
c) \[2.7\times 10^{2}J\]
d) \[3.4\times 10^{3}J\]
Explanation:
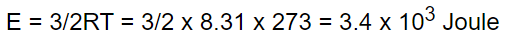
9. The average kinetic energy of hydrogen molecules at 300 K is E. At the same temperature, the average kinetic energy of oxygen molecules will
be
a) E/4
b) E/16
c) E
d) 4 E
Explanation: The average kinetic energy of hydrogen molecules at 300 K is E. At the same temperature, the average kinetic energy of oxygen molecules will be E
10. The average translational kinetic energy of a hydrogen gas molecules at NTP will be
[Boltzmann’s constant \[K_{B}=1.38\times 10^{-23}J\diagup K\] ]
a) \[0.186\times 10^{-20}Joule\]
b) \[0.372\times 10^{-20}Joule\]
c) \[0.56\times 10^{-20}Joule\]
d) \[5.6\times 10^{-20}Joule\]
Explanation: