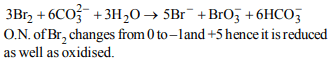1. Nitric oxide acts as a reducing agent in the reaction
a) \[4NH_{3}+5O_{2}\rightarrow 4NO+6H_{2}O\]
b) \[2NO+3I_{2}+4H_{2}O\rightarrow 2NO_3^-+6I^{-}+8H^{+}\]
c) \[2NO+H_{2}SO_{3}\rightarrow N_2O+H_{2}SO_{4}\]
d) \[2NO+H_{2}S\rightarrow N_2O+S+H_{2}O\]
Explanation: \[2NO+3I_{2}+4H_{2}O\rightarrow 2NO_3^-+6I^{-}+8H^{+}\]
2. What products are expected from the disproportionation
reaction of hypochlorous acid?
a) HCl and \[Cl_{2}O\]
b) HCl and \[HClO_{3}\]
c) \[HClO_{3}\] and \[Cl_{2}O\]
d) \[HClO_{2}\] and \[HClO_{4}\]
Explanation: HCl and \[HClO_{3}\]
3. Thiosulphate reacts differently with iodine and bromine in
the reactions given below:
\[2S_{2} O_3^{2-}+I_{2}\rightarrow S_{4} O_6^{2-}+2I^{-}\]
\[S_{2} O_3^{2-}+Br_{2}+5H_{2}O\rightarrow 2SO_4^{2-}+2Br^{-}+10H^{+}\]
Which of the following statements justifies the above dual
behaviour of thiosulphate?
a) Bromine is a stronger oxidant than iodine
b) Bromine is a weaker oxidant than iodine.
c) Thiosulphate undergoes oxidation by bromine and
reduction by iodine in these reactions.
d) Bromine undergoes oxidation and iodine undergoes
reduction in these reactions.
Explanation: Bromine is a stronger oxidant than iodine
4. Which of the following elements does not show
disproportionation tendency?
a) Cl
b) Br
c) F
d) I
Explanation: 'F'
5. \[\left(x\right)MnO_4^-+\left(y\right)H_{2}O_{2}\rightarrow 2Mn^{+2}+5H_{2}O+I3/2O_{2}+\left(z\right)e^{-}\]
In this reaction, value of (x), (y) and (z) are
a) 2, 5, 6
b) 5, 2, 9
c) 3, 5, 5
d) 2, 6, 6
Explanation: Value of (x), (y) and (z) are 2, 5, 6
6. In the following reaction, which is the species being oxidised ?
\[ 2Fe^{3+}\left(aq\right)+2I^{-}\left(aq\right) \rightarrow I_2 (aq) +2Fe^{2+}\left(aq\right)\]
a) \[ Fe^{3+}\]
b) \[ I^{-}\]
c) \[I_{2}\]
d) \[ Fe^{2+}\]
Explanation: O.N. of I- is –1 and in I2 O.N. is zero (loss of electrons). Hence I- oxidised
7. Which of the following reactions depicts the oxidising
property of \[SO_{2}\] ?
a) \[SO_{2}+H_{2}O\rightarrow H_{2}SO_{3}\]
b) \[2H_{2}S+SO_{2}\rightarrow 3S+2H_{2}O\]
c) \[Cl_{2}+SO_{2}\rightarrow SO_{2}Cl_{2}\]
d) \[ 2MnO_4^-+5SO_{2}+2H_{2}O\rightarrow 5SO_4^{2-}+2Mn^{2+}+4H^{+}\]
Explanation: SO2 oxidises H2 S to S, since the O.N. of S change from –2 to 0
8. Which of the following statements is not correct ?
a) Potassium permanganate is a powerful oxidising
substance.
b) Potassium permanganate is a weaker oxidising agent than
potassium dichromate
c) Potassium permanganate is a stronger oxidising agent
than potassium dichromate
d) Potassium dichromate oxidises a secondary alcohol into
a ketone.
Explanation:
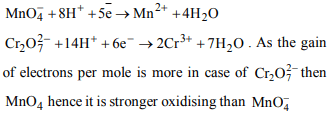
9. Which substance serves as reducing agent in the following reaction?
\[ 14H^++Cr_{2}O_7^{2-}+3Ni\rightarrow 2Cr^{3+} +7H_{2}O+3Ni^{2+}\]
a) \[H_{2}O\]
b) Ni
c) \[H^+\]
d) \[Cr_{2}O_7^{2-}\]
Explanation:

10. In the reaction
\[3Br_{2}+6CO_3^{2-}+3H_{2}O \rightarrow 5Br^{-}+BrO_3^- +6HCO_3^-\]
a) bromine is oxidised and carbonate is reduced.
b) bromine is reduced and water is oxidised
c) bromine is neither reduced nor oxidised
d) bromine is both reduced and oxidised
Explanation: