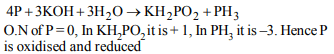1. The compound \[YBa_{2}Cu_{3}O_{7}\] which shows
superconductivity has copper in oxidation state ..........
Assume that the rare earth element Yttrium is in its usual +3
oxidation state
a) 3/7
b) 7/3
c) 3
d) 7
Explanation:
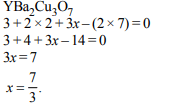
2. For \[H_{3}PO_{3}\] and \[H_{3}PO_{4}\] the correct choice is
a) \[H_{3}PO_{3}\] is dibasic and reducing
b) \[H_{3}PO_{3}\] is dibasic and non-reducing
c) \[H_{3}PO_{4}\] is tribasic and reducing
d) \[H_{3}PO_{3}\] is tribasic and non-reducing
Explanation:

3. The oxidation number of sulphur in \[S_{8},S_{2}F_{2},H_{2}S\] respectively, are
a) 0, +1 and –2
b) +2, +1 and –2
c) 0, +1 and +2
d) –2, +1 and –2
Explanation:
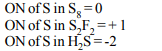
4. When \[SO_{2}\] is passed through acidified solution of potassium
dichromate, then chromium sulphate is formed. The change
in valency of chromium is
a) +4 to +2
b) +5 to +3
c) +6 to +3
d) +7 to +2
Explanation:
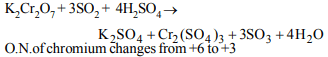
5. One mole of \[N_{2}H_{4}\] loses 10 moles of electrons to form a new
compound, y. Assuming that all nitrogen appear in the new
compound, what is the oxidation state of nitrogen in y (There
is no change in the oxidation state of hydrogen )
a) –1
b) –3
c) +3
d) +5
Explanation:

6. In the reaction : \[C+4HNO_{3}\rightarrow CO_{2}+2H_{2}O+4NO_{2}\]
HNO3 act as
a) an oxidizing agent
b) an acid
c) an acid as well as oxidizing agent
d) a reducing agent
Explanation: O.N. of C changes form 0 to + 4 by oxidation. Hence HNO3 is oxidising agent.
7. Which of the following reaction involves neither oxidation
nor reduction
a) \[CrO_4^{2-}\rightarrow Cr_{2}O_7^{2-}\]
b) \[Cr\rightarrow CrCl_{3}\]
c) \[Na\rightarrow Na^+\]
d) \[2S_{2}O_3^{2-}\rightarrow S_{4}O_6^{2-}\]
Explanation:

8. A compound of Xe and F is found to have 53.5% of Xe.
What is oxidation number of Xe in this compound ?
a) –4
b) 0
c) +4
d) +6
Explanation:
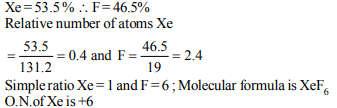
9. In the standardization of \[Na_{2}S_{2}O_{3}\] using \[K_{2}Cr_{2}O_{7}\] by
iodometry, the equivalent weight of \[K_{2}Cr_{2}O_{7}\] is
a) (molecular weight)/2
b) (molecular weight)/6
c) (molecular weight)/3
d) same as molecular weight
Explanation:
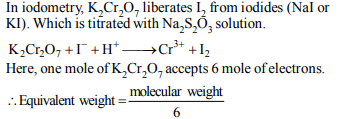
10.In the following reaction
\[4P + 3KOH + 3H_{2}O\rightarrow 3KH_{2}PO_{2} + PH_{3}\]
a) only phosphorus is oxidised and reduced
b) only phosphorus is reduced
c) phosphorus is both oxidised and reduced
d) phosphorus is neither oxidised nor reduced
Explanation: