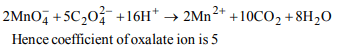1. In \[H_{2}O_{2}\] , the oxidation state of oxygen is
a) -2
b) -1
c) 0
d) -4
Explanation:
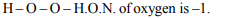
2. A, B and C are three elements forming a part of compound in
oxidation states of +2, +5 and –2 respectively. What could
be the compound ?
a) \[A_{2}\left(BC\right)_{2}\]
b) \[A_{2}\left(BC_{4}\right)_{3}\]
c) \[A_{3}\left(BC_{4}\right)_{2}\]
d) ABC
Explanation:
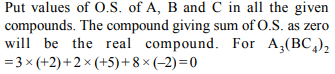
3.Among the following, identify the species with an atom in
+6 oxidation state
a) \[MnO_4^-\]
b) \[Cr \left(CN\right)_6^{3-}\]
c) \[NiF_6^{2-}\]
d) \[CrO_{2}Cl_{2}\]
Explanation:
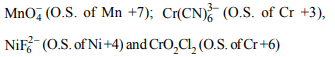
4. On reduction of \[KMnO_{4}\] by oxalic acid in acidic medium, the
oxidation number of Mn changes. What is the magnitude of
this change?
a) From 7 to 2
b) From 6 to 2
c) From 5 to 2
d) From 7 to 4
Explanation:
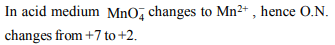
5. The oxidation number of iron in \[Fe_{3}O_{4}\] is
a) +2
b) +3
c) 8/3
d) 2/3
Explanation:
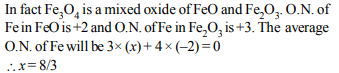
6. The oxidation number of S in \[H_{2}S_{2}O_{8}\] is
a) +2
b) +4
c) +6
d) +7
Explanation: O.N. of S in H2 S2O8 is + 6.
7. When KMnO4 acts as an oxidising agent and ultimately forms
\[MnO_4^{-2}, MnO_{2},Mn_{2}O_{3}\] and \[Mn^{+2}\] , then the number of
electrons transferred in each case respectively is
a) 4, 3, 1, 5
b) 1, 5, 3, 7
c) 1, 3, 4, 5
d) 3, 5, 7, 1
Explanation:
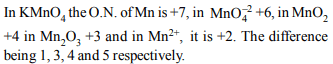
8. The oxidation number of sulphur in \[Na_{2}S_{4}O_{6}\] is
a) 1.5
b) 2.5
c) 3
d) 2
Explanation:
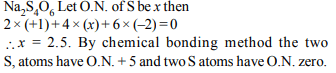
9.In which of the following reactions, there is no change in
valency ?
a) \[4KClO_{3} \rightarrow 3KClO_{4} + KCl\]
b) \[SO_{2} + 2H_{2}S \rightarrow 2H_{2}O + 3 S\]
c) \[BaO_{2} + H_{2}SO _{4} \rightarrow BaSO _{4}+ H_{2}O_{2}\]
d) \[2 BaO + O _{2}\rightarrow2 BaO_{2}\]
Explanation:
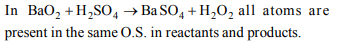
10. What is the coefficient of oxalate ion in the following
reaction ? \[MnO_4^-+ C _{2}O_4^{2-} + H^{+}\rightarrow Mn ^{2+}+CO_{2} + H _{2}O\]
a) 4
b) 2
c) 3
d) 5
Explanation: