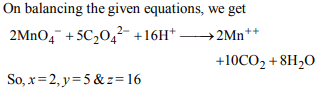1. \[\left(X\right) MnO_4^- + (Y)H_{2}O_{2}\rightarrow 2Mn^{+2} +5H_{2}O+9O_{2}+\left(Z\right)e^{-}\]
In this reaction, value of (X), (Y) and (Z) are
a) 2, 5, 6
b) 5, 2, 9
c) 3, 5, 5
d) 2, 6, 6
Explanation:
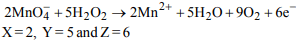
2.The oxidation number of phosphorus in pyrophosphoric
acid is
a) +3
b) +1
c) +4
d) +5
Explanation:

3. The oxidation states of sulphur in the anions \[SO_3^{2-},S_{2}O_4^{2-}\] and \[S_{2}O_6^{2-}\] follow the order
a) \[S_{2}O_6^{2-} < S_{2}O_4^{2-}< S_{2}O_3^{2-}\]
b) \[S_{2}O_4^{2-} < SO_3^{2-}< S_{2}O_6^{2-}\]
c) \[SO_3^{2-} < S_{2}O_4^{2-}< S_{2}O_6^{2-}\]
d) \[S_{2}O_4^{2-} < S_{2}O_6^{2-}< SO_3^{2-}\]
Explanation:
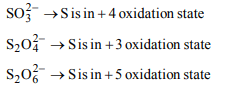
4. Oxidation numbers of P in \[PO_4^{3-}\] , of S in \[SO_4^{2-}\] and that of
Cr in \[Cr_{2}O_7^{2-}\] are respectively
a) + 3, + 6 and + 5
b) + 5, + 3 and + 6
c) – 3, + 6 and + 6
d) + 5, + 6 and + 6
Explanation:
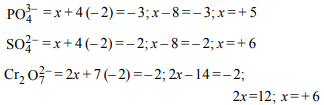
5. When \[Cl_{2}\] gas reacts with hot and concentrated sodium
hydroxide solution, the oxidation number of chlorine
changes from :
a) zero to +1 and zero to –5
b) zero to –1 and zero to +5
c) zero to –1 and zero to +3
d) zero to +1 and zero to –3
Explanation:
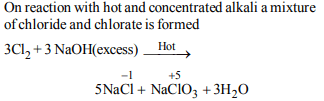
6. Which of the following is a redox reaction?
a) \[NaCl + KNO_{3} \rightarrow NaNO_{3} + KCl\]
b) \[CaC_{2}O_{4} + 2HCl \rightarrow CaCl_{2} + H_{2}C_{2}O_{4}\]
c) \[Mg(OH)_{2} + 2NH_{4}Cl \rightarrow MgCl_{2} + 2NH_{4}OH\]
d) \[Zn + 2AgCN \rightarrow 2Ag + Zn(CN)_{2}\]
Explanation:
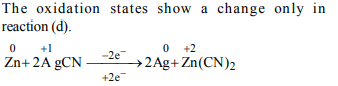
7. The oxidation state of chromium in the final product formed
by the reaction between KI and acidified potassium
dichromate solution is
a) + 3
b) +2
c) +6
d) +4
Explanation:
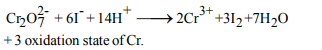
8. Which of the following chemical reactions depict the
oxidising beahviour of \[H_{2}SO_{4}\] ?
a) \[NaCl + H_{2}SO_{4}\rightarrow NaHSO _{4} + HCl\]
b) \[2PCl_{5} + H_{2}SO_{4}\rightarrow 2POCl _{3} + 2HCl + SO_{2}Cl_{2}\]
c) \[2HI + H_{2}SO_{4}\rightarrow I _{2} + SO_{2}+2H_{2}O\]
d) \[Ca\left(OH\right)_{2} + H_{2}SO_{4}\rightarrow CaSO _{4} + 2H_{2}O\]
Explanation:
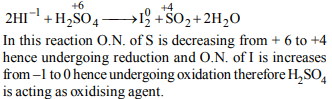
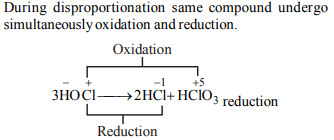
9. What products are expected from the disproportionation
reaction of hypochlorous acid?
a) HCl and \[ Cl_{2}O\]
b) HCl and \[ HClO_{3}\]
c) \[ HClO_{3}\] and \[ Cl_{2}O\]
d) \[ HClO_{2}\] and HClO4
Explanation:
10. Consider the following reaction :
\[ xMNO_4^-+yC_{2}O_4^{2-}+zH^{+}\rightarrow xMn^{2+}+2yCO_{2}+\frac{z}{2}H_{2}O\]
The value’s of x, y and z in the reaction are, respectively :
a) 5, 2 and 16
b) 2, 5 and 8
c) 2, 5 and 16
d) 5, 2 and 8
Explanation: