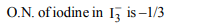1. Which of the following is a redox reaction ?
a) \[H_{2}SO_{4}\] with NaOH
b) In atomosphere, \[O_{3}\] from \[O_{2}\] by lightning
c) Nitrogen oxides from nitrogen and oxygen by lightning
d) Evaporation of \[H_{2}O\]
Explanation:

2. \[KMnO_{4}\] oxidises oxalic acid in acidic medium. The number of
\[CO_{2}\] molecules produced as per the balanced equation is
a) 10
b) 8
c) 6
d) 3
Explanation: 10 moles of CO2 are produced.
3. Of the following reactions, only one is a redox reaction.
Identify it
a) \[Ca(OH)_{2} + 2HCl\rightarrow CaCl_{2} + 2H_{2}O\]
b) \[BaCl_{2} + MgSO_{4} \rightarrow BaSO_{4} + MgCl_{2}\]
c) \[2S_{2}O_7^{2-}+ 2H_{2}O \rightarrow 4SO_4^{2-} +4H^{+}\]
d) \[Cu_{2}S+ 2FeO \rightarrow 2Cu + 2Fe +SO_{2}\]
Explanation:
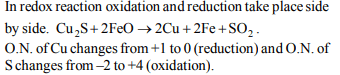
4.The reaction of \[KMnO_{4}\] and HCl results in
a) oxidation of Mn in \[KMnO_{4}\] and production of \[CI_{2}\]
b) reduction of Mn in \[KMnO_{4}\] and production of \[H_{2}\]
c) oxidation of Mn in \[KMnO_{4}\] and production of \[H_{2}\]
d) reduction of Mn in \[KMnO_{4}\] and production of \[CI_{2}\]
Explanation:
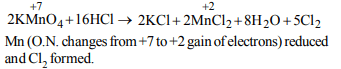
5. The reaction,
\[2H_{2} O\left(l\right)\rightarrow 4H^{+} (aq) +O_{2} \left(g\right) + 4e^{-}\]
is
a) an oxidation reaction
b) a reduction reaction
c) a redox reaction
d) a hydrolysis reaction
Explanation: Since there is loss of electrons, hence it is oxidation reaction
6. The chemical that undergoes self oxidation and self reduction
in the same reaction is
a) benzyl alcohol
b) acetone
c) formaldehyde
d) acetic acid
Explanation:
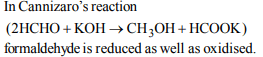
7. Phosphorus has the oxidation state of +3 in
a) phosphorous acid
b) orthophosphoric acid
c) hypophosphorous acid
d) metaphosphoric acid
Explanation:

8. In which of the compounds does 'manganese' exhibit highest
oxidation number ?
a) \[MnO _{2}\]
b) \[Mn _{3}O _{4}\]
c) \[K _{2}MnO _{4}\]
d) \[MnSO _{4}\]
Explanation: O.N. of Mn in K2MnO4 is +6 (find O.N. of Mn in others)
9. One of the following has both positive and negative oxidation
states
a) F
b) Cl
c) He
d) Na
Explanation: Cl has O. S. as –1, +1 +3 +5 and +7
10. In which of the following compounds, the oxidation number
of iodine is fractional ?
a) \[IF _{7}\]
b) \[I_3^-\]
c) \[IF _{5}\]
d) \[IF _{3}\]
Explanation: