1. In which of the following compounds, nitrogen has an
oxidation state of –1 ?
a) \[N _{2}O\]
b) \[NO_2^-\]
c) \[NH _{2}OH\]
d) \[N _{2}H_{4}\]
Explanation:
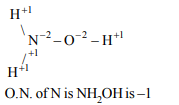
2. Which of the following involves transfer of five
electrons ?
a) \[MnO_4^-\rightarrow Mn^{2+}\]
b) \[CrO_4^{2-}\rightarrow Cr^{3+}\]
c) \[MnO_4^{2-}\rightarrow MnO_{2}\]
d) \[Cr_{2}O_7^{2-}\rightarrow 2Cr^{3+}\]
Explanation:
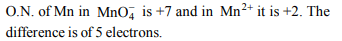
3. Oxidation number of nitrogen in \[\left(NH_{4}\right)_{2}SO_{4}\] is
a) –1/3
b) –1
c) +1
d) –3
Explanation:
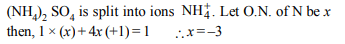
4. Oxidation number of carbon in \[CH_{2}CI_{2}\] is
a) –4
b) +4
c) 0
d) –2
Explanation:

5. Which of the following elements has least oxidation number?
a) \[Ni \left(CN\right)_{4}\]
b) \[Ni \left(CO\right)_{4}\]
c) \[Fe_{2}O_{3}\]
d) \[SF_{6}\]
Explanation: In metal carbonyls metal always has O.N. zero
6. The oxidation number of cobalt in \[K_{3}\left[Co \left(NO_{2}\right)_{6}\right]\] is
a) 0
b) +4
c) +3
d) +6
Explanation:
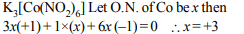
7. The brown ring complex is formulated as
\[\left[Fe \left(H_{2}O\right)_{5}NO\right]SO_{4}\] . The oxidation number of iron is
a) 1
b) 2
c) 3
d) 0
Explanation:
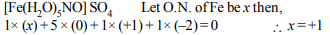
8. In which of the following complexes, oxidation state of metal
is zero ?
a) \[\left[Pt \left(NH_{3}\right)_{2}Cl_{2}\right]\]
b) \[\left[Cr \left(CO\right)_{6}\right]\]
c) \[\left[Cr \left(NH_{3}\right)_{3}Cl_{3}\right]\]
d) \[\left[Cr \left(en\right)_{2}Cl_{2}\right]\]
Explanation: Cr(CO)6 is metal carbonyl, hence O.N. of chromium is zero.
9. The oxidation state of chromium in potassium dichromate
\[\left(K_{2}Cr_{2}O_{7}\right)\] is
a) –5
b) +6
c) +2
d) -2
Explanation: K2Cr2O7 O.N. of Cr is +6
10. The oxidation state of osmium (Os) in \[OsO_{4}\] is
a) +7
b) +6
c) +4
d) +8
Explanation: OsO4 Let O.N. of Os be x then 1×(x) + 4(–2) = 0
x = 8