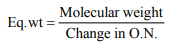1.The compound that can work both as an oxidising as well as
a reducing agent is
a) \[KMnO_4\]
b) \[H_{2}SO_{4}\]
c) \[BaO_{2}\]
d) \[H_{2}O_{2}\]
Explanation: In H2O2 the O.N. of O is –1 which can be increased to 0 or decresed to –2 hence H2O2 can work as reducting and oxidising agent
2. When \[SO_{2}\] is passed through the solution of potassium
iodate, the oxidation state of iodine changes from
a) + 5 to 0
b) + 5 to – 1
c) – 5 to 0
d) – 7 to – 1
Explanation:
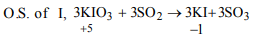
3. Which of the following behaves as both oxidising and
reducing agents ?
a) \[H_{2}SO_{4}\]
b) \[SO_{2}\]
c) \[H_{2}O\]
d) \[HNO_{3}\]
Explanation: In SO2 the O.N. of S can increase and decrease. Hence can behave as reducing and oxidising agent. Oxidation state of S varies from –2 to 6.
4. In the reaction
\[2FeCl_{3} + H_{2}S\rightarrow 2FeCl_{2} + 2HCl + S\]
a) \[FeCl_{3} \] acts as an oxidising agent
b) Both \[ H_{2}S\] are \[FeCl_{3} \] are oxidised
c) \[FeCl_{3} \] is oxidised while \[ H_{2}S\] is reduced
d) \[ H_{2}S\] acts as an oxidising agent
Explanation:
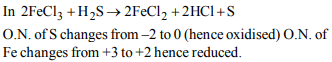
5. Which of the following is not a reducing agent?
a) \[ SO_{2}\]
b) \[ H_{2}O_{2}\]
c) \[ CO_{2}\]
d) \[ NO_{2}\]
Explanation: In CO2 , O.N. of C is +4 which is maximum. So it can act as oxidising agent and not as reducing agent.
6. Number of moles of \[ K_{2}Cr_{2}O_{7}\] reduced by one mole of Sn2+
ions is
a) 1/3
b) 3
c) 1/6
d) 6
Explanation:
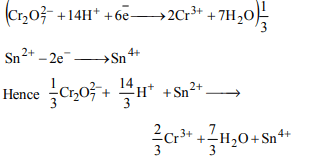
7. The reaction in which hydrogen peroxide acts as a reducing
agent is
a) \[ PbS + 4 H_{2}O_{2} \rightarrow PbSO_{4} + 4H_{2}O\]
b) \[ 2KI + H_{2}O_{2} \rightarrow 2KOH + I_{2}\]
c) \[ 2FeSO_{4} + H_{2}SO_{4} +H_{2}O_{2}\rightarrow Fe_{2}\left(SO_{4}\right)_{3} +2H_{2}O\]
d) \[ Ag_{2}O + H_{2}O_{2} \rightarrow 2Ag +H_{2}O+ O_{2}\]
Explanation: In Ag2O (O.N. of Ag +1) in Ag the O.N. is 0. There is gain of electrons, hence H2O2 is reducing
8. \[ HNO_{3} \] acts as
a) acid
b) oxidising agent
c) reducing agent
d) Both (a) and (b)
Explanation: In HNO3 O.N. of N is +5 which is maximum value hence it can act as oxidising agent and as an acid only.
9. When \[ KMnO_{4} \] reacts with acidified \[ FeSO_{4} \]
a) \[ FeSO_{4} \] is oxidised and \[ KMnO_{4} \] is reduced
b) only \[ KMnO_{4} \] is oxidised
c) only \[ FeSO_{4} \] is oxidised
d) None of these
Explanation:
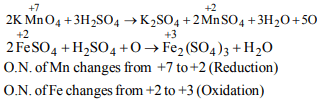
10. Formula weight divided by the change in oxidation number
gives
a) equivalent weight of an oxidant
b) equivalent weight of the reductant
c) the number of electrons gained in the reaction
d) the equivalent weight of the oxidant or reductant
Explanation: