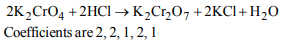1. The equivalent weight of Mohr’s salt,
\[ FeSO_{4} \left(NH_{4}\right)_{2}SO_{4}.6H_{2}O\]
is equal to
a) its molecular weight
b) its atomic weight
c) half-its molecular weight
d) one-third its molecular weight
Explanation:
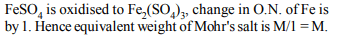
2. In the reaction between \[SO_{2}\] and \[O_{3}\] the equivalent weight of
ozone is
a) the same as its molecular weight
b) half of the molecular weight
c) one-third of the molecular weight
d) one-fourth of the molecular weight
Explanation:
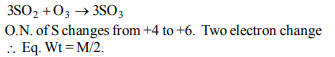
3. Equivalent weight of iron in \[Fe_{2}O_{3}\] would be
a) 28
b) 56
c) 18.6
d) 112
Explanation:
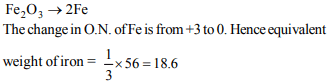
4.Atomic number of an element is 22. The highest O.S. exhibited
by it in its compounds is
a) 1
b) 2
c) 3
d) 4
Explanation:

5. The equivalent weight of potassium permaganate in acid
solution is
a) 158
b) 31.6
c) 52.16
d) 79
Explanation: (158/5 = 31.6)
6. The equivalent weight of phosphoric acid \[\left(H_{3}PO_{4}\right)\] in the
reaction:\[NaOH+ H_{3}PO_{4} \rightarrow NaH_{2}PO_{4} +H_{2}O\]
a) 59
b) 49
c) 25
d) 98
Explanation:
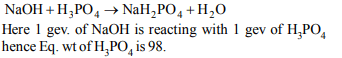
7. Equivalent mass of oxidising agent in the reaction,
\[SO_{2}+2H_{2}S\rightarrow 3S+2H_{2}O\]
is
a) 32
b) 64
c) 16
d) 8
Explanation:
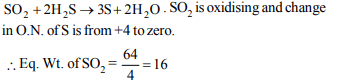
8. In the chemical reaction, \[K_{2}Cr_{2}O_{7}+XH_{2}SO _{4}+YSO_{2}\rightarrow K_{2}SO_{4}+Cr_{2}\left(SO_{4}\right)_{3} +Z H_{2}O\]
X, Y and Z are
a) 1, 3, 1
b) 4, 1, 4
c) 3, 2, 3
d) 2, 1, 2
Explanation:
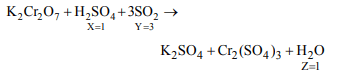
9. Consider the following reaction
\[5H_{2}O_{2}+XClO_{2}+2OH^{-}\rightarrow XCl^{-}+YO_{2}+6H_{2}O\]
The reaction is balanced if
a) X = 5, Y = 2
b) X = 2, Y = 5
c) X = 4, Y = 10
d) X = 5, Y = 5
Explanation:
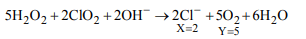
10. The set of numerical coefficients that balances the equation
\[K_{2}CrO_{4}+HCl\rightarrow K_{2}Cr_{2}O_{7}+KCl+H_{2}O\]
a) 1, 1, 2, 2, 1
b) 2, 2, 1, 1, 1
c) 2, 1, 1, 2, 1
d) 2, 2, 1, 2, 1
Explanation: