1. PbF4, PbCl4 exist but PbBr4 and PbI4 do not exist because of
a) large size of Br– and I–
b) strong oxidising character of \[Pb^{4+}\]
c) strong reducing character of \[Pb^{4+}\]
d) low electronegativity of Br– and I–.
Explanation:
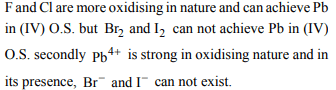
2. The element that does not form a monoxide is
a) lead
b) tin
c) germanium
d) silicon
Explanation: Silicon does not form mono oxide.
3. Pyrosilicate ion is
a) \[SiO_2^{2-}\]
b) \[SiO_4^{2-}\]
c) \[Si_{2}O_6^{7-}\]
d) \[Si_{2}O_7^{6-}\]
Explanation:

4. Freon -12 is used as a
a) refrigerant
b) insecticide
c) fungicide
d) herbicide
Explanation: Freon -12 is used as refrigerant
5. Which one of the following allotropic forms of carbon is
isomorphous with crystalline silicon?
a) Graphite
b) Coal
c) Coke
d) Diamond.
Explanation: Diamond and crystalline silicon are isomorphous.
6. The structure and hybridization of \[Si\left(CH_{3}\right)_{4}\] is
a) Bent, sp
b) Trigonal,\[sp^{2}\]
c) Octahedral,\[d^{2}sp^{3}\]
d) Tetrahedral,\[sp^{3}\]
Explanation:
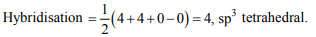
7. Silicon is an important constituent of
a) rocks
b) minerals
c) alloys
d) vegetables.
Explanation: SiO2 , hence silicon, is an important constituent of rocks.
8. In laboratory, silicon can be prepared by the reaction of
a) SiO2 with Mg
b) by heating C in electric furnace
c) by heating potassium fluorosilicate with potassium
d) None of these
Explanation:
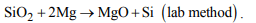
9. Silica is soluble in
a) HCl
b) \[HNO_{3}\]
c) \[H_{2}SO_{4}\]
d) HF
Explanation:
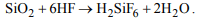
10. Quartz is a crystalline variety of
a) Si
b) \[SiO_{2}\]
c) \[Na_{2}SiO_{3}\]
d) SiC
Explanation: Purest form of SiO2 is quartz