1. Which of the following oxides is strongly basic ?
a) \[B_{2}O_{3}\]
b) \[Al_{2}O_{3}\]
c) \[Ga_{2}O_{3}\]
d) \[Tl_{2}O_{3}\]
Explanation:
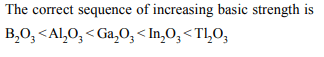
2. Which out of the following compounds does not exist?
a) \[BF_{3}\]
b) \[TlCl_{3}\]
c) \[TlCl_{5}\]
d) Both (b) and (c)
Explanation: Because Tl+5 does not exist
3. Which of the following does not give a borax bead
test ?
a) Chromium
b) Ferrous salt
c) Sodium
d) Cobalt
Explanation: Colourless salt or ion (ex. Na) will not give a borax bead test.
4. Borazole is known as
a) organic benzene
b) organic xylene
c) inorganic benzene
d) inorganic xylene
Explanation: Borazole is known as inorganic benzene
5. The \[I.E_{1}\] among the group 13 member follows as
a) B > Al < Ga < Tl
b) B > Al > Ga > Tl
c) B > Ga > Al > Tl
d) B > Ga < Al < Tl
Explanation: The IE1 of Ga is more than that of Al because of the small atomic size and greater effective nuclear charge of Ga
6. The melting pt. of group 13 follows the order
a) B > Al > Ga > In > Tl
b) B > Al < Ga > In > Tl
c) B > Al > Tl > In > Ga
d) B > Al < Ga < In < Tl
Explanation: Due to structural changes, melting point, increases from Ga to Tl and Ga has the lowest melting point
7. The compounds of boron and hydrogen are collectively
called
a) diboranes
b) borazoles
c) boracits
d) boranes
Explanation: The compounds of boron and hydrogen are collectively called boranes
8. The bonds present in borazole or inorganic benzene are
a) \[9\sigma,6\pi\]
b) \[12\sigma,3\pi\]
c) \[6\sigma,9\pi\]
d) \[15\sigma\] only
Explanation: \[12\sigma,3\pi\]
9. The aluminium salt commonly used to stop bleeding is
a) aluminium sulphate
b) potash alum
c) aluminium chloride
d) aluminium fluoride
Explanation: potash alum
10. The nature of the solution of Potash alum is
a) basic
b) acidic
c) neutral
d) amphoteric
Explanation: The nature of the solution of Potash alum is acidic