1. Which of the following is/ are methanide (s) ?
a) \[Be_{2}C\]
b) \[Al_{4}C_{3}\]
c) \[Mg_{2}C_{3}\]
d) Both (a) and (b)
Explanation: Be2 C and Al4 C3 give methane with water
2. Which of the following statements is false?
a) Water gas is a mixture of hydrogen and carbon monoxide
b) Producer gas is a mixture of CO and nitrogen
c) Water gas is a mixture of water vapour and hydrogen
d) Natural gas consists of methane, ethane and gaseous
hydrocarbons
Explanation: Water gas is CO + H2
3. Which gas is essential constituent of almost all fuel gases ?
a) \[CO_{2}\]
b) \[N_{2}\]
c) CO
d) \[H_{2}O\]
Explanation: CO is essential constituent of almost all fuel gases.
4. Which does not exist
a) \[\left[SnCl_{6}\right]^{2-}\]
b) \[\left[GeCl_{6}\right]^{2-}\]
c) \[\left[SiCl_{6}\right]^{2-}\]
d) \[\left[CCl_{6}\right]^{2-}\]
Explanation: Carbon, due to absence of d-orbitals can not extend its coordination number beyond four.
5.Newly shaped glass articles when cooled suddenly become
brittle, therefore these are cooled slowly, this process in
known as
a) tempering
b) annealing
c) quenching
d) galvanising
Explanation: Annealing makes the glass soft.
6. Mark the correct statement
a) Water gas is used in manufacture of methyl alcohol
b) Water gas has highest calorific value
c) Water gas burns with luminous flame
d) The production of water gas is exothermic process
Explanation:
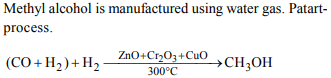
7. \[H_{2}SO_{4}\] is not used for preparation of \[CO_{2}\] from marble chips
because
a) it does not react
b) huge amount of heat is evolved
c) the reaction is vigorous
d) calcium sulphate is sparingly soluble and gets deposited
on marble chips and stops the reaction.
Explanation: Sparingly soluble CaSO4 deposits on marble and stops the reaction
8. \[CO_{2}\] is used for extinguishing fire because
a) it has a relatively high critical temperature
b) in solid state, it is called dry ice
c) it is neither combustible nor a supporter of combustion
d) it is a colourless gas
Explanation: CO2 is incombustible and non supporter of combustion
9. Lead pipes are not suitable for drinking water because
a) lead forms basic lead carbonate
b) lead reacts with water containing air to form \[Pb\left(OH\right)_{2}\]
c) a layer of lead dioxide is deposited over pipes
d) lead reacts with air to form litharge
Explanation:
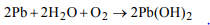
10. \[CO_{2}\] and \[N _{2}\] are non-supporters of combustion. However for
putting out fires \[CO_{2}\] is preferred over \[N _{2}\] because \[CO_{2}\]
a) does not burn
b) forms non-combustible products with burning substances
c) is denser than nitrogen
d) is a more reactive gas
Explanation: CO2 being more dense covers the igniting material more effectively than N2 .