1. \[B_{2}O_{3}\] is
a) acidic
b) basic
c) amphoteric
d) None of these
Explanation: \[B_{2}O_{3}\] is acidic
2. Boric acid is polymeric due to
a) its acidic nature
b) the presence of hydrogen bonds
c) its monobasic nature
d) its geometry
Explanation:
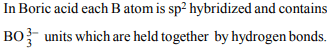
3. \[B\left(OH\right)_{3}\] is
a) monobasic acid
b) dibasic acid
c) tribasic acid
d) triacidic base
Explanation: \[B\left(OH\right)_{3}\] is monobasic acid
4. Which of the following hydroxide is acidic ?
a) \[Al\left(OH\right)_{3}\]
b) \[Ca\left(OH\right)_{3}\]
c) \[Tl\left(OH\right)_{3}\]
d) \[B\left(OH\right)_{3}\]
Explanation:
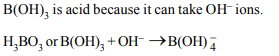
5. \[BF_{3}\] acts as an acid according to the concept of
a) Lewis
b) Bronsted
c) Arrhenius
d) None of these
Explanation: Since BF3 is an electron deficient molecule (according to lewis concept)
6. Which of the following is electron deficient ?
a) \[NH_{3}\]
b) \[BCl_{3}\]
c) \[PCl_{3}\]
d) None of these
Explanation: \[BCl_{3}\]
7. \[NH_{3}\] and \[BF_{3}\] form an adduct readily because they form
a) a coordinate bond
b) a hydrogen bond
c) an ionic bond
d) a covalent bond
Explanation: \[NH_{3}\] and \[BF_{3}\] form an adduct readily because they form a coordinate bond
8. \[BF_{3}\] is used as a catalyst in several industrial processes due to its
a) strong reducing nature
b) weak reducing action
c) strong Lewis acid nature
d) weak Lewis acid character
Explanation: \[BF_{3}\] is used as a catalyst in several industrial processes due to its weak Lewis acid character
9. Among the halides of the elements of group 13 the one which is most acidic is
a) \[BF_{3}\]
b) \[AlCl_{3}\]
c) \[BCl_{3}\]
d) \[BBr_{3}\]
Explanation: \[BBr_{3}\]
10. In diborane
a) 4–bridged hydrogens and two terminal hydrogens are present
b) 2– bridged hydrogens and four terminal hydrogens are present
c) 3–bridged and three terminal hydrogens are present
d) None of these
Explanation: 2– bridged hydrogens and four terminal hydrogens are present