1. Name of the structure of silicates in which three oxygen atoms
of \[\left[SiO_{4}\right]^{4-}\] are shared
a) Pyrosilicate
b) Sheet silicate
c) Linear chain silicate
d) Three dimensional silicate
Explanation:

2. When \[PbO_{2}\] reacts with conc. \[HNO_{3}\] the gas evolved is
a) \[NO_{2}\]
b) \[O_{2}\]
c) \[N_{2}\]
d) \[N_{2}O\]
Explanation:
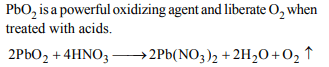
3. The species present in solution when \[CO_{2}\] is dissolved in
water are
a) \[CO_{2},H_{2}CO_{3},HCO_3^-,CO_3^{2-}\]
b) \[H_{2}CO_{3},CO_3^{2-}\]
c) \[CO_3^{2-},HCO_3\]
d) \[CO_{2},H_{2}CO_{3}\]
Explanation:
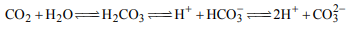
4. On addition of excess of sodium hydroxide solution to
stannous chloride solution, we obtain :
a) \[Sn\left( OH\right)_{2}\]
b) \[SnO_{2}.H_{2}O\]
c) \[Na_{2}SnO_{2}\]
d) None of these
Explanation:
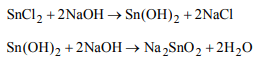
5. Lead pipes are readily corroded by
a) \[H_{2}SO_{4}\]
b) HCl
c) \[CH_{3}COOH\]
d) pure water
Explanation: Lead pipes are readily corroded by water containing organic acids
6. A salt which gives \[CO_{2}\] with hot \[H_{2}SO_{4}\] and also decolourises
acidified \[KMnO_{4}\] on warming is
a) bicarbonate
b) carbonate
c) oxalate
d) acetate
Explanation:
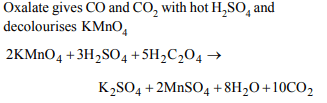
7. Which halide is least stable and has doubtful existence
a) \[CI_{4}\]
b) \[GeI_{4}\]
c) \[SnI_{4}\]
d) \[PbI_{4}\]
Explanation: In nature Pb4+ is strong oxidant and I- is strong reductant. Hence PbI4 cannot exist.
8. \[R_{3}SiCl\] on hydrolysis forms
a) \[R_{3}SiOH\]
b) \[R_{3}Si -O-SiR_{3}\]
c) \[R_{2}Si = O\]
d) None of these
Explanation:
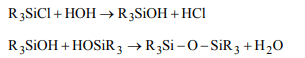
9. Which melts in boiling water ?
a) Gun metal
b) Wood’s metal
c) Monel metal
d) Bell metal
Explanation: Wood metal m.p.t. 70ºC .
10. Incomplete combustion of petrol or diesel oil in automobile
engines can be best detected by testing the fuel gases for
the presence of
a) \[CO + H_{2}O\]
b) CO
c) \[NO_{2}\]
d) \[SO_{2}\]
Explanation: The exhaust of auto gives CO due to incomplete combustion of petrol or diesel.