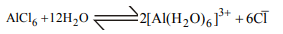1. Aluminium vessels should not be washed with materials
containing washing soda because
a) washing soda is expensive
b) washing soda gets easily decomposed
c) washing soda reacts with aluminium to form soluble
aluminate
d) washing soda reacts with aluminium to form insoluble
aluminium oxide
Explanation: Aluminium vessels should not be washed with materials containing washing soda because washing soda reacts with aluminium to form soluble aluminate
2. Which out of the following is potash alum ?
a) \[K_{2}SO_{4}.Al_{2}(SO_{4})_{3}.24H_{2}O\]
b) \[K_{2}SO_{4}.Cr_{2}(SO_{4})_{3}.24H_{2}O\]
c) \[K_{2}SO_{4}.Fe_{2}(SO_{4})_{3}.24H_{2}O\]
d) \[\left[NH_{4}\right]_{2}SO_{4}.FeSO_{4}.6H_{2}O\]
Explanation: \[K_{2}SO_{4}.Al_{2}(SO_{4})_{3}.24H_{2}O\]
3. In borax bead test which compound is formed?
a) Ortho-borate
b) Meta-borate
c) Double oxide
d) Tetra-borate
Explanation:
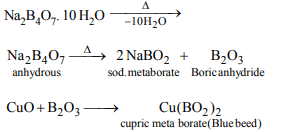
4. \[Al_{2}O_{3}\] can be converted to anhydrous \[AlCl_{3}\] by heating
a) \[Al_{2}O_{3}\] with NaCl in solid state
b) a mixture of \[Al_{2}O_{3}\] and carbon in dry \[Cl_{2}\] gas
c) \[Al_{2}O_{3}\] with \[Cl_{2}\] gas
d) \[Al_{2}O_{3}\] vwith HCl gas
Explanation: Al2O3 can be converted to anhydrous AlCl3 by heating a mixture of Al2O3 and carbon in dry Cl2
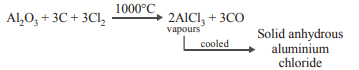
5. The tendency of BF3, BCl3 and BBr3 to behave as Lewis acid
decreases in the sequence:
a) \[BCl_{3}>BF_{3}>BBr_{3}\]
b) \[BBr_{3}>BCl_{3}>BF_{3}\]
c) \[BBr_{3}>BF_{3}>BCl_{3}\]
d) \[BF_{3}>BCl_{3}>BBr_{3}\]
Explanation: p-p overlap between B and F is maximum due to identical size and energy of p-orbitals, so electron deficiency in boron of BF3 is neutralized partially to the maximum extent by back donation. Hence BF3 is least acidic. As the size of halogen atom increases from F to I, the extent of overlap between 2p-orbital of B and a bigger p-orbital of halogen decreases. Therefore the electron deficiency of B increases.
6. Aluminium is extracted from alumina \[\left(Al_{2}O_{3}\right)\] by electrolysis
of a molten mixture of :
a) \[Al_{2}O_{3}+ HF + NaAlF_{4}\]
b) \[Al_{2}O_{3}+ CaF_{2} + NaAlF_{4}\]
c) \[Al_{2}O_{3} + Na_{3}AlF_{6}+ CaF_{2}\]
d) \[Al_{2}O_{3}+ KF + Na_{3}AlF_{6}\]
Explanation: Fused alumina (Al2O3 ) is a bad conductor of electricity. Therefore, cryolite (Na3AlF6 ) and fluorspar (CaF2 ) are added to purified alumina which not only make alumina a good conductor of electricity but also reduce the melting point of the mixture to around 1140 K.
7. Which of the following structure is similar to graphite?
a) B
b) \[ B_{4}C\]
c) \[ B_{2}H_{6}\]
d) BN
Explanation: Boron nitride (BN) is known as inorganic graphite. The most stable form is hexagonal one. It has layered structure similar to graphite
8. Alum helps in purifying water by
a) forming Si complex with clay partiles
b) sulphate part which combines with the dirt and removes
it
c) coagulaing the mud particles
d) making mud water soluble
Explanation: Alum furnishes Al3+ ions which bring about coagulation of negatively charged clay particles, bacteria etc
9. Aluminium is extracted by the electrolysis of
a) bauxite
b) alumina
c) alumina mixed with molten cryolite
d) molten cryolite.
Explanation: Alumina is mixed with cryolite which acts as an electrolyte
10. Aluminium chloride exists as dimer, \[ Al_{2}Cl_{6}\] in solid state as
well as in solution of non-polar solvents such as benzene.
When dissolved in water, it gives
a) \[ \left[Al\left(OH\right)_{6}\right]^{3-}+3HCl\]
b) \[ \left[Al\left(H_{2}O\right)_{6}\right]^{3+}+3Cl^{-}\]
c) \[ Al^{3+}+3Cl^{-}\]
d) \[ Al_{2}O_{3}+6HCl\]
Explanation: