1. Graphite is a soft solid lubricant extremely difficult to melt.
The reason for this anomalous behaviour is that graphite
a) is an allotropic form of diamond
b) has molecules of variable molecular masses like polymers
c) has carbon atoms arranged in large plates of rings of
strongly bound carbon atoms with weak interplate bonds
d) is a non-crystalline substance
Explanation: Statement (c) is correct
2. Glass is a
a) super-cooled liquid
b) gel
c) polymeric mixture
d) micro-crystalline solid
Explanation: Glass is a transparent or translucent super cooled liquid
3. For making good quality mirrors, plates of float glass are used.
These are obtained by floating molten glass over a liquid metal
which does not solidify before glass. The metal used can be
a) tin
b) sodium
c) magnesium
d) mercury
Explanation: It is because mercury exists as liquid at room temperature
4. The soldiers of Napolean army while at Alps during freezing
winter suffered a serious problem as regards to the tin buttons
of their uniforms. White metallic tin buttons got converted
to grey powder. This transformation is related to
a) a change in the partial pressure of oxygen in the air
b) a change in the crystalline structure of tin
c) an interaction with nitrogen of the air at very low
temperature
d) an interaction with water vapour contained in the humid
air
Explanation:
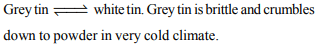
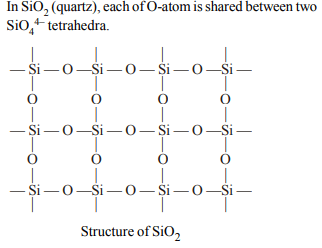
5. In silicon dioxide
a) there are double bonds between silicon and oxygen
atoms
b) silicon atom is bonded to two oxygen atoms
c) each silicon atom is surrounded by two oxygen atoms
and each oxygen atom is bonded to two silicon atoms
d) each silicon atom is surrounded by four oxygen atoms
and each oxygen atom is bonded to two silicon atoms
Explanation:
6. A metal, M forms chlorides in its +2 and +4 oxidation states.
Which of the following statements about these chlorides is
correct?
a) \[MCl_{2}\] is more ionic than \[MCl_{4}\]
b) \[MCl_{2}\] is more easily hydrolysed than \[MCl_{4}\]
c) \[MCl_{2}\] is more volatile than \[MCl_{4}\]
d) \[MCl_{2}\] is more soluble in anhydrous ethanol than \[MCl_{4}\]
Explanation: Metal atom in the lower oxidation state forms the ionic bond and in the higher oxidation state the covalent bond because higher oxidation state means small size and great polarizing power and hence greater the covalent character. Hence MCl2 is more ionic than MCl4 .
7. The stability of dihalides of Si, Ge, Sn and Pb increases
steadily in the sequence
a) \[PbX_{2} << SnX_{2} << GeX2 << SiX_{2}\]
b) \[GeX_{2} << SiX_{2} << SnX _{2} << PbX _{2}\]
c) \[SiX_{2} << GeX_{2} << PbX _{2} << SnX _{2}\]
d) \[SiX_{2} << GeX_{2} << SnX _{2} << PbX _{2}\]
Explanation: Reluctance of valence shell electrons to participate in bonding is called inert pair effect. The stability of lower oxidation state (+2 for group 14 element) increases on going down the group. So the correct order is SiX2 < GeX2 < SnX2 < PbX2
8. In context with the industrial preparation of hydrogen from
water gas \[\left(CO + H _{2}\right)\] , which of the following is the correct
statement?
a) CO is oxidised to \[ CO _{2}\] with steam in the presence of a
catalyst followed by absorption of of \[ CO _{2}\] in alkali
b) CO is removed by absorption in aqueous \[ Cu _{2}Cl _{2}\]
solution
c) \[ H _{2}\] is removed through occlusion with pd
d) CO and \[ H _{2}\] , are fractionally separated using differences
in their densities
Explanation:
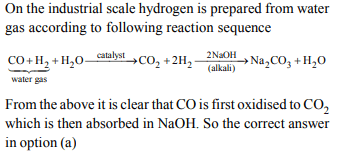
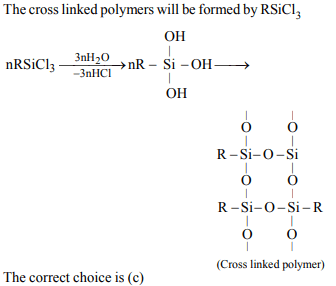
9. Among the following substituted silanes the one which will
give rise to cross linked silicone polymer on hydrolysis is
a) \[ R _{4}Si\]
b) \[ R _{2}SiCl _{2}\]
c) \[ RSiCl _{3}\]
d) \[ R _{3}SiCl \]
Explanation:
10. In view of the signs of \[\triangle_{r}G^{\circ}\] for the following reactions :
\[PbO_{2} + Pb\rightarrow 2PbO\] \[\triangle_{r}G^{\circ}< 0\]
\[SnO_{2} + Sn\rightarrow 2SnO\] \[\triangle_{r}G^{\circ}>0\]
Which oxidation states are more characteristics for lead and tin ?
a) For lead + 2, for tin + 2
b) For lead + 4, for tin + 4
c) For lead + 2, for tin + 4
d) For lead + 4, for tin + 2
Explanation:
