1. An oxide of lead which is used in lead storage battery, in
safety matches and is a powerful oxidising agent is
a) PbO
b) \[PbO_{2}\]
c) \[Pb_{3}O_{4}\]
d) \[2PbO.PbO_{2}\]
Explanation: \[PbO_{2}\]
2. Lead sulphate is soluble in
a) conc. \[HNO_{3}\]
b) conc. HCl
c) solution of ammonium acetate
d) water
Explanation:
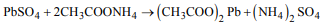
3.The percentage of lead in lead pencil is
a) zero
b) 20
c) 80
d) 70
Explanation: Lead pencils do not contain lead. Lead pencils contain graphite.
4. The important ore of lead is
a) chalcopyrites
b)haematite
c) galena
d) bauxite
Explanation: Galena PbS.
5. Which of the following lead oxides is present in ‘Sindhur’?
a) PbO
b) \[PbO_{2}\]
c) \[Pb_{2}O_{3}\]
d) \[Pb_{3}O_{4}\]
Explanation: \[Pb_{3}O_{4}\] is also known as Sindhur
6. Which of the following reactions occurs at the cathode during
the charging of a lead storage battery?
a) \[Pb^{2+} + 2e^{-} \rightarrow Pb\]
b) \[Pb^{2+} + SO_4^{2-} \rightarrow PbSO_{4}\]
c) \[Pb\rightarrow Pb^{2+} + 2e^{-} \]
d) \[PbSO_{4}+2H_{2}O\rightarrow PbO_{2}+ 4H^{+} +SO_4^{2-} + 2e^{-} \]
Explanation: \[PbSO_{4}+2H_{2}O\rightarrow PbO_{2}+ 4H^{+} +SO_4^{2-} + 2e^{-} \]
7. White lead is
a) \[Pb_{3}O_{4}\]
b) PbO
c) \[2PbCO_{3}.Pb\left(OH\right)_{2} \]
d) \[Pb\left(CH_{3}COO\right)_{2}.Pb\left(OH\right)_{2} \]
Explanation:

8. The oxide which cannot act as reducing agent is
a) \[SO_{2} \]
b) \[NO_{2} \]
c) \[CO_{2} \]
d) \[ClO_{2} \]
Explanation: CO2, C is in +4 O.S. which is maximum
9. A solid element (symbol Y) conducts electricity and forms
two chlorides \[YCl_{n} \] (colourless volatile liquid) and \[YCl_{n-2} \] (a colourless solid). To which one of the following groups of
the periodic table does Y belong?
a) 13
b) 14
c) 15
d) 16
Explanation: SnCl4 is colourless volatile liquid and SnCl2 is colourless solid Sn conducts electricity and it belongs to 14 group
10. Which of the following bonds has the most polar
character?
a) C-O
b) C-Br
c) C-S
d) C-F
Explanation: C-F is most polar due to highest electronegativity difference.