1. Although Al has a high oxidation potential it resists
corrosion because of the formation of a tough, protective
coat of
a) \[Al\left(NO_{3}\right)_{3}\]
b) AlN
c) \[Al_{2}O_{3}\]
d) \[Al_{2}\left(CO_{3}\right)_{3}\]
Explanation: \[Al_{2}O_{3}\]
2. \[AlCl_{3}\] on hydrolysis gives
a) \[Al_{2}O_{3}.H_{2}O\]
b) \[Al_{2}O_{3}\]
c) \[Al\left(OH_{3}\right)\]
d) \[AlCl_{3}.6H_{2}O\]
Explanation: \[AlCl_{3}\] on hydrolysis gives \[Al\left(OH_{3}\right)\]
3. Action of caustic soda on aluminium hydroxide gives a
compound having formula
a) \[Al_{2}\left(OH\right)_{4}\]
b) \[Na_{2}Al\left(OH\right)_{4}\]
c) \[NaAlO_{2}\]
d) \[Na_{3}AlO_{3}\]
Explanation:
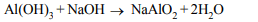
4. \[Al\left(OH\right)_{3}\] is
a) acidic
b) basic
c) amphoteric
d) neither acidic nor basic
Explanation: \[Al\left(OH\right)_{3}\] is amphoteric
5. A lake can be obtained by making a mixture of a coloured
dye with
a) \[NH_{4}OH\]
b) \[Ba\left(OH\right)_{2}\]
c) \[Al\left(OH\right)_{3}\]
d) NaOH
Explanation: \[Al\left(OH\right)_{3}\]
6. \[AlCl_{3}\] is
a) anhydrous and covalent
b) anhydrous and ionic
c) covalent and basic
d) coordinate and acidic
Explanation: \[AlCl_{3}\] is anhydrous and covalent
7. Anhydrous \[AlCl_{3}\] is prepared from
a) conc. HCl and Al metal
b) aluminium and \[Cl_{2}\]
c) dry HCl gas + heated Al metal
d) dil. HCl and Al metal
Explanation:
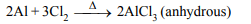
8. Aluminium chloride is a/an
a) Bronsted - Lowry acid
b) Arrhenius acid
c) Lewis acid
d) Lewis base
Explanation: Aluminium chloride is a/an lewis acid
9. Which member of group 13 does not exhibit the group
valency in its compounds ?
a) Boron
b) Aluminium
c) Gallium
d) Thallium
Explanation: Gallium
10. The highly toxic element of group 13 is
a) Al
b) B
c) Ga
d) Tl
Explanation: The highly toxic element of group 13 is Tl