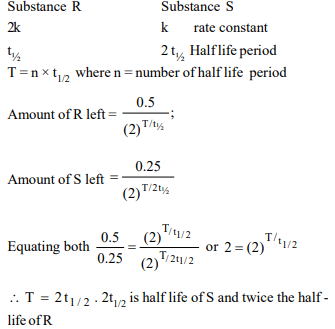1.The time for half life period of a certain reaction
\[A\rightarrow\] Products is 1 hour. When the initial concentration
of the reactant ‘A’, is 2.0 mol L–1, how much time does it take
for its concentration to come from 0.50 to 0.25 mol \[L^{-1}\] if it is
a zero order reaction ?,
a) 4 h
b) 0.5 h
c) 0.25 h
d) 1 h
Explanation:
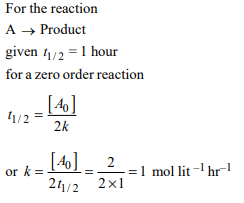
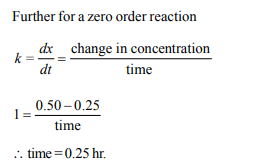
2. Consider the reaction :
\[Cl_{2}\left(aq\right)+H_{2}S\left(aq\right)\rightarrow S\left(s\right)+2H^{+}\left(aq\right)+2Cl^{-}\left(aq\right)\]
The rate equation for this reaction is
\[rate = k \left(Cl_{2}\right)\left[H_{2}S\right]\]
Which of these mechanisms is/are consistent with this rate equaion?
A.\[Cl_{2}+H_{2}S\rightarrow H^{+}+Cl^{-}+Cl^{+}+HS^{-}\left(slow\right)\]
\[Cl^{+}+HS^{-}\rightarrow H^{+}+Cl^{-}+S \left(fast\right)\]
B. \[H_{2}S\rightleftharpoons H^{+}+HS^{-}\] (fast equilibrium)
\[Cl_{2}+HS^{-}\rightarrow 2Cl^{-}+H^{+}+S \left(Slow\right)\]
a) B only
b) Both A and B
c) Neither A nor B
d) A only
Explanation:
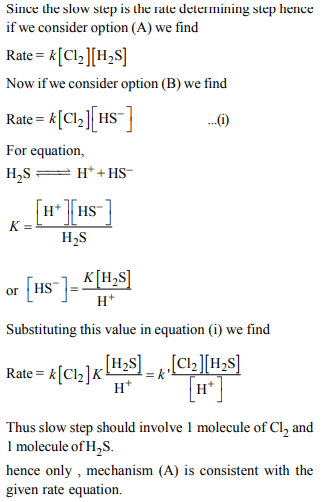
3. A reactant (A) froms two products :
\[A \rightarrow B\] , Activation Energy \[Ea_{1}\]
\[A \rightarrow C\] , Activation Energy \[Ea_{2}\]
If \[Ea_{2}= 2 Ea_{1}\] , then \[K_{1}\] and \[K_{2}\] are related as
a) \[K_{2}= K_{1}^{Ea_{1}/RT}\]
b) \[K_{2}= K_{1}^{Ea_{2}/RT}\]
c) \[K_{1}=A K_{2}e^{Ea_{1}/RT}\]
d) \[K_{1}=2K_{2}e^{Ea_{2}/RT}\]
Explanation:
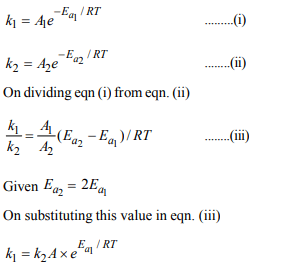
4. The rate of a chemical reaction doubles for every 10°C rise of
temperature. If the temperature is raised by 50°C, the rate of
the reaction increases by about :
a) 10 times
b) 24 times
c) 32 times
d) 64 times
Explanation:

5.For a first order reaction \[\left(A\right)\rightarrow\] products the concentration of
A changes from 0.1 M to 0.025 M in 40 minutes.
The rate of reaction when the concentration of A is 0.01 M is :
a) \[1.73 × 10^{-5} M/min\]
b) \[3.47 × 10^{-4} M/min\]
c) \[3.47 × 10^{-5} M/min\]
d) \[1.73 × 10^{-4} M/min\]
Explanation:
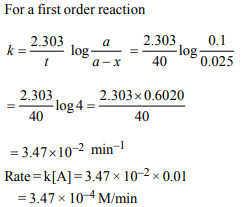
6. The rate of a reaction doubles when its temperature changes
from 300 K to 310 K. Activation energy of such a reaction will
be : (R = 8.314 J \[K^{-1} mol^{-1}\] and log 2 = 0.301)
a) 53.6 kJ \[mol^{-1}\]
b) 48.6 kJ \[mol^{-1}\]
c) 58.5 kJ \[mol^{-1}\]
d) 60.5 kJ \[mol^{-1}\]
Explanation:
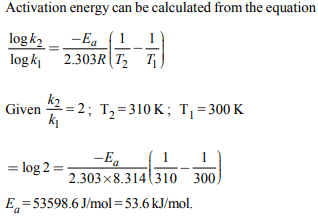
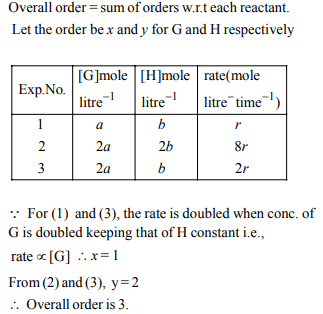
7. Consider a reaction \[aG+bH\rightarrow\] Products. When
concentration of both the reactants G and H is doubled, the
rate increases by eight times. However, when concentration
of G is doubled keeping the concentration of H fixed, the rate
is doubled. The overall order of the reaction is
a) 0
b) 1
c) 2
d) 3
Explanation:
8. Under the same reaction conditions, initial concentration of
1.386 mol \[dm^{-3}\] of a substance becomes half in 40 seconds
and 20 seconds through first order and zero order kinetics,
respectively. Ratio \[\left(K_{1}/K_{0}\right)\]) of the rate constant for first order
\[\left(K_{1}\right)\] and zero order \[\left(K_{0}\right)\] of the reaction is
a) 0.5 \[mol^{-1} dm^{3}\]
b) 1.0 mol \[dm^{-3}\]
c) 1.5 mol \[dm^{-3}\]
d) 2.0 \[mol^{-1} dm^{3}\]
Explanation:
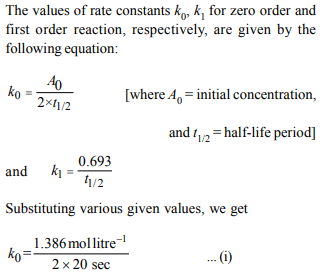
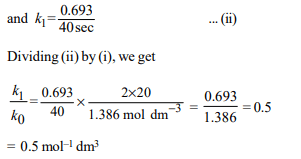
9. For a first order reaction \[A \rightarrow P\] , the temperature (T) dependent
rate constant (k) was found to follow the equation
log \[k = – (2000)\frac{1}{T}+6.0\]
The pre-exponential factor A and
the activation energy Ea, respectively, are
a) \[1.0 × 10^{6} s^{-1}\] and 9.2 kJ \[mol^{-1}\]
b) 6.0 \[s^{-1}\] and 16.6 kJ \[mol^{-1}\]
c) \[1.0 × 10^{6} s^{-1}\] and 16.6 kJ \[mol^{-1}\]
d) \[1.0 × 10^{6} s^{-1}\] and 38.3 kJ \[mol^{-1}\]
Explanation:
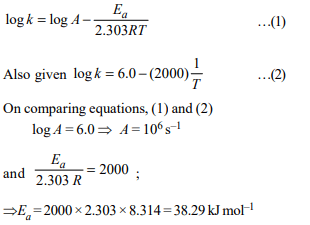
10. Two substances R and S decompose in solution
independently, both following first order kinetics. The rate
constant of R is twice that of S. In an experiment, the solution
initially contained 0.5 millimoles of R and 0.25 millimoles of
S. The molarities of R and S will be equal just at the end of
time equal to
a) twice the half life of R
b) twice the half life of S
c) the half life of S
d) the half life of R
Explanation: