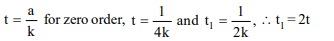1. For a first-order reaction, the half life period is independent
of
a) initial concentration
b) cube root of initial concentration
c) first power of final concentration
d) square root of final concentration
Explanation: t1/2 for 1st order is independent of initial concentration
2. For a first order reaction, the plot of log K against 1/T is a
straight line. The slope of the line is equal to
a) \[-\frac{E_{a}}{R}\]
b) \[-\frac{2.303}{E_{a}R}\]
c) \[-\frac{E_{a}}{2.303}\]
d) \[\frac{-E_{a}}{2.303R}\]
Explanation:
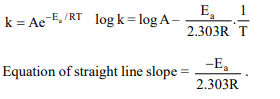
3. For a first order reaction, a plot of log (a – x) against time is
a straight line with a negative slope equal to
a) \[\frac{-K}{2.303}\]
b) – 2.303 k
c) \[\frac{2.303}{K}\]
d) \[-\frac{E_{a}}{2.303 R}\]
Explanation:
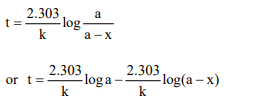
4. A first order reaction does not depend upon
a) volume
b) pressure
c) temperature
d) All of these
Explanation: Order of reaction is independent of given factors
5. \[2A\rightarrow B+C\] , would be a zero order reaction when
a) the rate of reaction is proportional to square of conc. of A
b) the rate of reaction remains same at any conc. of A
c) the rate remains unchanged at any conc. of B and C
d) the rate of reaction doubles if conc. of B is increased to
double
Explanation: For zero order the rate does not change with concentration
6. Which of the following is correct for a first order reaction?
a) \[t_{1/2}\propto a\]
b) \[t_{1/2}\propto 1/a\]
c) \[t_{1/2}\propto a^{0}\]
d) \[t_{1/2}\propto 1/a^{2}\]
Explanation:

7. Order of reaction is decided by
a) temperature
b) mechanism of reaction as well as relative concentration
of reactants
c) molecularity
d) pressure
Explanation: The order of a chemical reaction is given by concentration of reactants appearing in the lowest step
8. Half-life of a reaction is found to be inversely proportional to
the cube of initial concentration. The order of reaction is
a) 4
b) 3
c) 5
d) 2
Explanation:
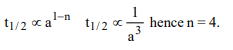
9. The reaction \[L\rightarrow M\] is started with 10.0 g of L. After 30
and 90 minutes 5.0 g and 1.25 g of L respectively are left. The
order of the reaction is
a) 0
b) 1
c) 2
d) 3
Explanation:

10. If initial concentration is reduced to 1/4th in a zero order
reaction, the time taken for half the rection to complete
a) remains same
b) becomes 4 times
c) becomes one-fourth
d) doubles
Explanation: