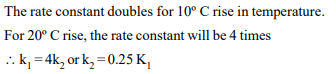1. The rate of a gaseous reaction is given by the expression
k(A)(B). If the volume of the reaction vessel is suddenly
reduced to 1/4 of the initial vol. the reaction rate relative to
the original rate will be
a) \[\frac{1}{16}\]
b) \[\frac{1}{8}\]
c) 8
d) 16
Explanation:

2.The units for the rate constant of first order reaction is
a) \[s^{-1}\]
b) mol \[L^{-1} S^{-1}\]
c) mol \[S^{-1}\]
d) L \[mol ^{-1} S^{-1}\]
Explanation:

3.The rate constant of reaction depends upon
a) temperature
b) pressure
c) volume
d) All the these
Explanation: Rate constant depends upon temperature
4. The rate constant of a reaction is 10.8 × \[10^{-5}\] mol \[dm ^{-3} s^{-1}\] . The order of the reaction is
a) zero
b) 1
c) 2
d) 3
Explanation: mol dm–3 s–1 units are for zero order
5. If concentration of reactants is increased by 'x', then k
becomes
a) \[ln\frac{K}{X}\]
b) \[\frac{K}{X}\]
c) \[K+X\]
d) K
Explanation: Rate constant does not change with concentration
6. Rate constant in the case of first order reaction is
a) inversely proportional to the concentration units
b) independent of concentration units
c) directly proportional to the concentration units
d) inversely proportional to the square of the concentration
units
Explanation: k = time–1 for 1st order. It is independent of concentration terms.
7.The Arrhenius equation expressing the effect of temperature
on the rate constant of the reaction is
a) \[K=e^{-E_{a}/RT}\]
b) \[K=\frac{E_{a}}{RT}\]
c) \[K=log_{e}\frac{E_{a}}{RT}\]
d) \[K=Ae^{-E_{a}/RT}\]
Explanation:
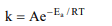
8. For the following homogeneous reaction,
\[A+B\rightarrow C\]
the unit of rate constant is
a) \[Sec^{-1}\]
b) \[Sec^{-1}mol L^{-1}\]
c) \[Sec^{-1}mol^{-1}L\]
d) \[Sec^{-1}mol^{-2} L^{2}\]
Explanation:
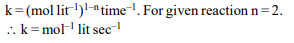
9. In Arrhenius plot, intercept is equal to
a) \[-\frac{E_{a}}{R}\]
b) ln A
c) ln K
d) \[log_{10}a\]
Explanation:

10. A chemical reaction was carried out at 300 K and 280 K. The
rate constants were found to be \[K_{1}\] and \[K_{2}\] respectively. then
a) \[K_{1}=4K_{1}\]
b) \[K_{2}=2K_{1}\]
c) \[K_{2}=0.25K_{1}\]
d) \[K_{2}=0.5K_{1}\]
Explanation: