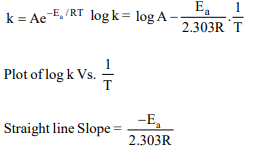1. Which of the following has been used to explain the subject
of chemical kinetics
a) Collision theory of bimolecular reactions
b) The activated complex theory
c) Arrhenius equation
d) All of these
Explanation: All the statements are correct
2. Which of the following statements is incorrect?
a) Activation energy for the forward reaction equals to
activation energy for the reverse reaction
b) For a reversible reaction, an increase in temperature
increases the reaction rate for both the forward and the
backward reaction
c) The larger the initial reactant concentration for a second
order reaction, the shorter is its half-life
d) When \[\triangle t\] is infinitesimally small, the average rate equals
the instantaneous rate
Explanation:
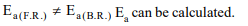
3.Activation energy of a chemical reaction can be determined
by
a) evaluating rate constant at standard temperature
b) evaluating velocities of reaction at two different
temperatures
c) evaluating rate constants at two different temperatures
d) changing concentration of reactants
Explanation:
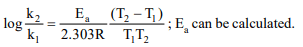
4. A catalyst increases rate of reaction by
a) decreasing enthalpy
b) decreasing internal energy
c) decreasing activation energy
d) increasing activation energy
Explanation: Activation energy is lowered in presence of +ve catalyst
5. The activation energy for a simple chemical reaction \[A\rightarrow B\]
is \[E_{a}\] in forward direction. The activation energy for reverse
reaction
a) is always less than \[E_{a}\]
b) can be less than or more than \[E_{a}\]
c) is always double of \[E_{a}\]
d) is negative of \[E_{a}\]
Explanation: Since the nature of reaction (i.e. exothermic or endothermic) not given therefore Ea for reverse reaction can be more or less.
6. Activation energy of the reaction is
a) the energy released during the reaction
b) the energy evolved when activated complex is formed
c) minimum amount of energy needed to overcome the
potential barrier
d) the energy needed to form one mole of the product
Explanation: Activation energy of the reaction is minimum amount of energy needed to overcome the potential barrier
7. The activation energy for a hypothetical
reaction, \[A\rightarrow\] Product, is 12.49 kcal/mole. If temperature is
raised from 295 to 305, the rate of reaction increased by
a) 60%
b) 100%
c) 50%
d) 20%
Explanation: For 10°C rise of temperature the rate is almost doubled.
8. In a reaction, the threshold energy is equal to
a) activation energy + normal energy of reactants
b) activation energy - normal energy of reactants
c) normal energy of reactants - activation energy
d) average kinetic energy of molecules of reactants
Explanation: Threshold Energy = Energy of activation + Internal energy
9.When two reactants A and B are mixed to give products C
and D, the reaction quotient, 'Q', at the initial stages of the
reaction
a) is zero
b) decreases with time
c) is independent of time
d) increases with time
Explanation:
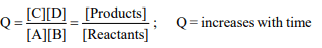
10. The temperature dependence of rate constant (k) of a
chemical reaction is written in terms of Arrhenius equation, k
\[= A . e^{-E_{a}}\] Activation energy \[\left(E_{a}\right)\] of the reaction can be
calculated by plotting
a) k vs. \[\frac{1}{log T}\]
b) log k vs. \[\frac{1}{ T}\]
c) log k vs. \[\frac{1}{log T}\]
d) k vs. T
Explanation: