1. For the reaction \[\left[N_{2}O_{5}\left(g\right)\rightarrow 2NO_{2}\left(g\right)+1/2 O_{2}\left(g\right)\right]\]
the value of rate of disappearance of \[N_{2}O_{5}\] is given as
\[6.25 × 10^{-3}\] mol \[L^{-1}s^{-1}\] . The rate of formation of \[NO_2\] and \[O_{2}\] is given respectively as :
a) \[6.25 × 10^{-3}\] mol \[L^{-1}s^{-1}\] and \[6.25 × 10^{-3}\] mol \[L^{-1}s^{-1}\]
b) \[1.25 × 10^{-2}\] mol \[L^{-1}s^{-1}\] and \[3.125 × 10^{-3}\] mol \[L^{-1}s^{-1}\]
c) \[6.25 × 10^{-3}\] mol \[L^{-1}s^{-1}\] and \[3.125 × 10^{-3}\] mol \[L^{-1}s^{-1}\]
d) \[1.25 × 10^{-2}\] mol \[L^{-1}s^{-1}\] and \[6.25 × 10^{-3}\] mol \[L^{-1}s^{-1}\]
Explanation:
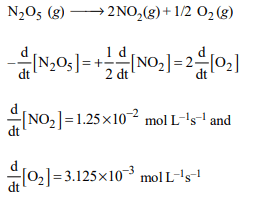
2. For an endothermic reaction, energy of activation is \[E_{a}\] and
enthalpy of reaction of \[\triangle H\] (both of these in kJ/mol). Minimum
value of \[E_{a}\] will be.
a) less than \[\triangle H\]
b) equal to \[\triangle H\]
c) more than \[\triangle H\]
d) equal to zero
Explanation: equal to \[\triangle H\]
3. The rate of the reaction \[2NO+ Cl_{2}\rightarrow 2NOCl\] is given
by the rate equation rate \[=K\left[ NO\right]^{2}\left[Cl_{2}\right]\]
The value of the rate constant can be increased by:
a) increasing the concentration of NO.
b) increasing the temperature
c) increasing the concentration of the \[Cl_{2}\]
d) doing all of these
Explanation:
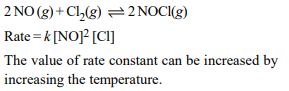
4. Which one of the following statements for the order of a
reaction is incorrect ?
a) Order can be determined only experimentally.
b) Order is not influenced by stoichiometric coefficient of
the reactants
c) Order of reaction is sum of power to the concentration
terms of reactants to express the rate of reaction
d) Order of reaction is always whole number.
Explanation: order of reaction may be zero, whole number or fractional.
5. The rate of the reaction \[2N_{2}O_{5}\rightarrow 4NO_{2}+2O_{2}\] can be written
in three ways :
\[\frac{-d\left[N_{2}O_{5}\right]}{dt}=K\left[N_{2}O_{5}\right]\]
\[\frac{d\left[NO_{2}\right]}{dt}=K'\left[N_{2}O_{5}\right]\]
\[\frac{d\left[O_{2}\right]}{dt}=K''\left[N_{2}O_{5}\right]\]
The relationship between k and k' and between k and k'' are :
a) \[K'=2K; K'=K\]
b) \[K'=2K; K''=K/2\]
c) \[K'=2K; K''=2K\]
d) \[K'=K; K''=K\]
Explanation:
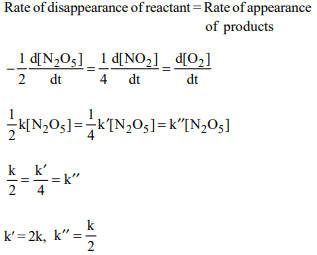
6. The unit of rate constant for a zero order reaction is
a) mol \[L^{-1} s^{-1}\]
b) L \[mol^{-1} s^{-1}\]
c) \[ L^{2} mol^{-2} s^{-1}\]
d) \[s^{-1}\]
Explanation:
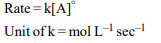
7. In a zero-order reaction for every 10° rise of temperature, the
rate is doubled. If the temperature is increased from 10°C to
100°C, the rate of the reaction will become :
a) 256 times
b) 512 times
c) 64 times
d) 128 times
Explanation:
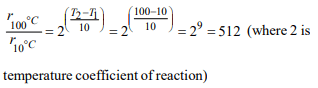
8. Activation energy \[\left(E_{a}\right)\] and rate constants \[(K_{1}\] and \[K_{2})\] of a
chemical reaction at two different temperatures \[(T_{1}\] and \[T_{2})\]
are related by :
a) \[ln \frac{K_{2}}{K_{1}}=-\frac{E_{a}}{R}\left(\frac{1}{T_{1}}-\frac{1}{T_{2}}\right)\]
b) \[ln \frac{K_{2}}{K_{1}}=-\frac{E_{a}}{R}\left(\frac{1}{T_{2}}-\frac{2}{T_{1}}\right)\]
c) \[ln \frac{K_{2}}{K_{1}}=\frac{E_{a}}{R}\left(\frac{1}{T_{1}}-\frac{1}{T_{2}}\right)\]
d) Both b and c
Explanation:
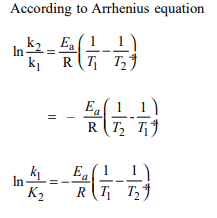
9. What is the activation energy for a reaction if its rate doubles
when the temperature is raised from 20°C to 35°C? (R = 8.314 J \[mol^{-1} K^{-1})\]
a) 269 kJ \[mol^{-1}\]
b) 34.7 kJ \[mol^{-1}\]
c) 15.1 kJ \[mol^{-1}\]
d) 342 kJ \[mol^{-1}\]
Explanation:
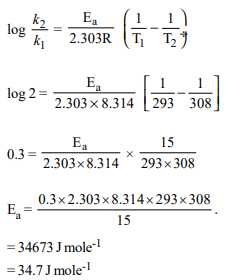
10. The formation of gas at the surface of tungsten due to
adsorption is the reaction of order
a) 0
b) 1
c) 2
d) insufficient data
Explanation: It is zero order reaction