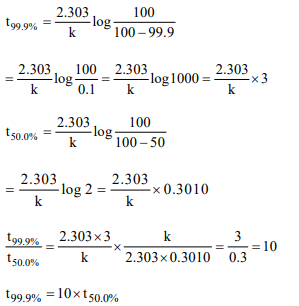1. For a reaction, the rate constant at particular temperature
has a value of \[2.0 × 10^{-3}\] mol \[lit^{-1}s^{-1}\] . The order of the reaction
is
a) -1
b) 0
c) 1
d) 2
Explanation:

2.For the gas phase decomposition A \[\rightarrow\] 2B, the rate
constant is \[ 6.93 × 10^{-3} min^{-1}\] at 300 K. The percentage of a
remaining at the end of 300 minutes is
a) 75
b) 50
c) 25
d) 1.25
Explanation:
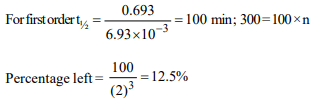
3. For a first order reaction \[t_{0.75}\] is 1368 seconds, therefore,
the specific rate constant in \[ sec^{-1}\] is
a) \[ 10^{-3}\]
b) \[ 10^{-2}\]
c) \[ 10^{-9}\]
d) \[ 10^{-5}\]
Explanation:
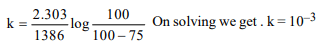
4. Consider the following reactions at 300 K
\[X\rightarrow Y\] (uncatalysed reaction)
\[X\rightarrow Y\] (catalysed reaction)
The energy of activation is lowered by 0.314 KJ \[mol^{-1}\] for
the catalysed reaction. The rate of reaction is
a) 38 times
b) 15 times
c) 25 times
d) 22 times that of uncatalysed reaction
Explanation:
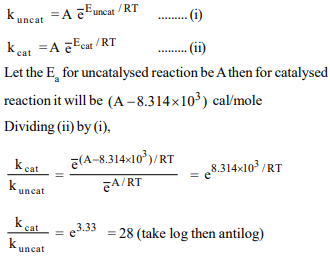
5. For a reaction \[ A\rightarrow B\] , the rate increases by a factor of 2.25
when the concentration of A is increased by 1.5. What is the
order of the reaction?
a) 3
b) 0
c) 2
d) 1
Explanation:
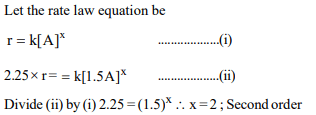
6. The rate constant , the activation energy and the Arrhenius
parameter of a chemical reaction at 25°C are \[3×10^{-4} s^{-1}\] ,104.4kJ /mol and \[6×10^{14} s^{-1}\] respectively. The value of the
rate constant as \[T\rightarrow \infty\] is
a) \[2.0×10^{18} s^{-1}\]
b) \[6.0×10^{14} s^{-1}\]
c) Infinity
d) \[3.6×10^{30} s^{-1}\]
Explanation:
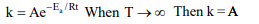
7. A substance ‘A’ decomposes in solution following first order
kinetics. Flask I contains l L of a 1M solution of A and flask
II contains 100 ml of a 0.6 M solution. After 8 hours the
concentration of A in flask I has become 0.25. What will be
the time taken for concentration of A in flask II to become
0.3M ?
a) 0.4 h
b) 2.4 h
c) 4.0 h
d) Can’t be calculated since rate constant is not given
Explanation:
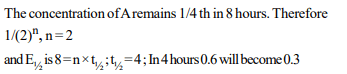
8. A reaction rate constant is given by
\[k =1.2\times 10^{14} e^{-25000/RT}sec^{-1}\] . It means
a) log k versus log T will give a straight line with a slope as
–25000
b) log k versus T will give a straight line with slope as
25000
c) log k versus log 1/T will give a straight line with slope as
–25000
d) log k versus 1/T will give a straight line
Explanation:
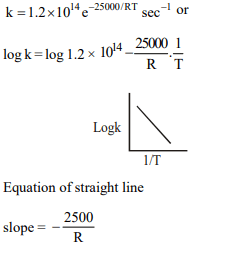
9. Consider a general chemical change \[2A +3B \rightarrow\] products.
The rate with respect to A is \[r_{1}\] and that with respect to B is
\[r_{2}\] .The rates \[r_{1}\] and \[r_{2}\] are related as
a) \[3r_{1} = 2r_{2}\]
b) \[r_{1} = r_{2}\]
c) \[2r_{1} = 3r_{2}\]
d) \[r_1^2 =2r_2^2\]
Explanation:
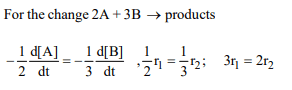
10. In case of first order reaction,the ratio of the time required for
99.9% completion to 50% completion is
a) 2
b) 5
c) 10
d) None of these
Explanation: