1.A first order reaction is half-completed in 45 minutes. How
long does it need for 99.9% of the reaction to be completed?
a) 20 hours
b) 10 hours
c) \[7\frac{1}{2} hours\]
d) 5 hours
Explanation:
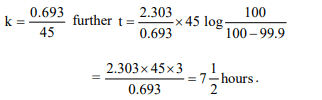
2. A reaction which is of first order w.r.t. reactant A, has a rate
constant 6 \[min ^{-1}\] . If we start with [A] = 0.5 mol \[L^{-1}\] , when
would [A] reach the value of 0.05 mol \[L^{-1}\]
a) 0.384 min
b) 0.15 min
c) 3 min
d) 3.84 min
Explanation:
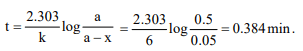
3. The rate constant for the reaction
\[2N_{2}O_{5}\rightarrow 4NO_{2}+O_{2}\]
is \[3.0 × 10^{-5} sec^{-1}\] . If the rate is
\[2.40 × 10^{-5}\] mol \[litre^{-1} sec^{-1}\] , then the concentration of \[N_{2}O_{5}\] (in mol \[litre^{-1})\] is
a) 1.4
b) 1.2
c) 0.04
d) 0.8
Explanation:
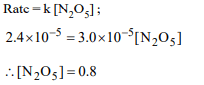
4. The reaction, \[N_{2}O_{5}\left(CCl_{4} \right) \rightarrow 2NO_{2}+\frac{1}{2}O_{2}\left(g\right)\]
is first order in \[N_{2}O_{5}\] with rate constant \[6.2 × 10^{-4} s^{-1}\]
What
is the value of rate of reaction when \[\left[N_{2}O_{5}\right]\] = 1.25 mol \[L^{-1}\] ?
a) \[7.75 × 10^{-4}\] mol \[L^{-1} S^{-1}\]
b) \[6.35 × 10^{-3}\] mol \[L^{-1} S^{-1}\]
c) \[5.15 × 10^{-5}\] mol \[L^{-1} S^{-1}\]
d) \[3.85 × 10^{-4}\] mol \[L^{-1} S^{-1}\]
Explanation:
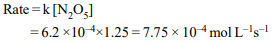
5. The time taken for 90% of a first order reaction to complete
is approximately
a) 1.1 times that of half-life
b) 2.2 times that of half-life
c) 3.3 times that of half-life
d) 4.4 times that of half-life
Explanation:
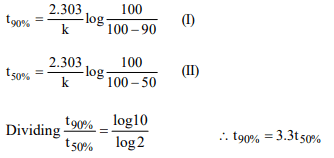
6. A substance initial concentration (a) reacts according to
zero order kinetics. What will be the time for the reaction to
go to completion
a) \[\frac{a}{K}\]
b) \[\frac{K}{a}\]
c) \[\frac{a}{2K}\]
d) \[\frac{2K}{a}\]
Explanation: For zero order reaction tcompletion = a/k.
7. The reaction \[2N_{2}O_{5}\rightleftharpoons 2N_{2}O_{4}+O_{2}\]
a) bimolecular and of second order
b) unimolecular and of first order
c) bimolecular and of first order
d) bimolecular and of zero order
Explanation:

8. The given reaction
\[2FeCl_{3}+SnCl_{2}\rightleftharpoons 2FeCl_{2}+SnCl_{4}\]
is an example of
a) first order reaction
b) second order reaction
c) third order reaction
d) None of these
Explanation: Third order
9. Collision theory is applicable to
a) first order reactions
b) zero order reactions
c) bimolecular reactions
d) intra-molecular reactions
Explanation: Applicable to bimolecular reactions.
10. According to the collision theory of reaction rates, the rate
of reaction increases with temperature due to
a) greater number of collision
b) higher velocity of reacting molecules
c) greater number of molecules having the activation
energy
d) decrease in the activation energy
Explanation: greater number of collision