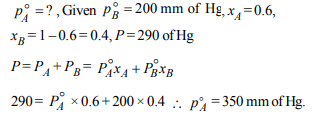1. For which of the following parameters the structural isomers
\[C_{2}H_{5} OH\] and \[CH_{3}OCH_{3} \] would be expected to have the same
values? (Assume ideal behaviour)
a) Boiling points
b) Vapour pressure at the same temperature
c) Heat of vapourization
d) Gaseous densities at the same temperature and pressure
Explanation: Gaseous densities of ethanol and dimethyl ether would be same at same temperature and pressure. The heat of vaporisation, V.P. and b.pt. will differ due to H - bonding in ethanol.
2. Which of the following liquid pairs shows a positive
deviation from Raoult’s law ?
a) Water - nitric acid
b) Benzene - methanol
c) Water - hydrochloric acid
d) Acetone - chloroform
Explanation: A mixture of benzene and methanol show positive deviation from Raoult’s law
3. Which one of the following statements is FALSE?
a) The correct order of osmotic pressure for 0.01 M
aqueous solution of each compound is
\[ BaCl_{2} > KCl > CH_{3}COOH > Sucrose \]
b) The osmotic pressure \[\left(\pi\right)\] of a solution is given by the
equation \[\pi\] = MRT, where M is the molarity of the solution
c) Raoult’s law states that the vapour pressure of a
component over a solution is proportional to its mole
fraction
d) Two sucrose solutions of same molality prepared in
different solvents will have the same freezing point
depression
Explanation:
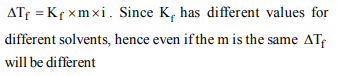
4. If \[\alpha\] is the degree of dissociation of \[Na_{2}SO_{4}\] , the Vant
Hoff’s factor (i) used for calculating the molecular mass is
a) \[1 – 2 \alpha\]
b) \[1 + 2 \alpha\]
c) \[1 – \alpha\]
d) \[1 + \alpha\]
Explanation:
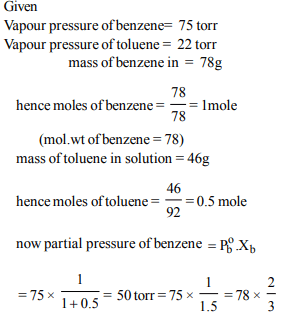
5. Benzene and toluene form nearly ideal solution. At 20°C, the
vapour pressure of benzene is 75 torr and that of toluene is
22 torr. The partial vapour pressure of benzene at 20°C for a
solution containing 78 g of benzene and 46 g of toluene in
torr is
a) 53.5
b) 37.5
c) 25
d) 50
Explanation:
6. Two solutions of a substance (non electrolyte) are mixed in
the following manner. 480 ml of 1.5 M first solution + 520 ml
of 1.2 M second solution. What is the molarity of the final
mixture ?
a) 2.70 M
b) 1.344 M
c) 1.50 M
d) 1.20 M
Explanation:
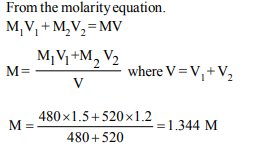
7. Equimolar solutions in the same solvent have
a) different boiling and different freezing points
b) same boiling and same freezing points
c) same freezing point but different boiling point
d) same boiling point but different freezing point
Explanation: Equimolar solutions of normal solutes in the same solvent will have the same b. pts and same f. pts.
8. Density of a 2.05M solution of acetic acid in water is
1.02 g/mL. The molality of the solution is
a) 2.28 mol \[kg^{-1}\]
b) 0.44 mol \[kg^{-1}\]
c) 1.14 mol \[kg^{-1}\]
d) 3.28 mol \[kg^{-1}\]
Explanation:
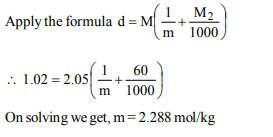
9. 18 g of glucose \[\left(C_{6}H_{12}O_{6}\right)\] is added to 178.2 g of water. The
vapour pressure of water for this aqueous solution at 100ºC
is
a) 76.00 Torr
b) 752.40 Torr
c) 759.00 Torr
d) 7.60 Torr
Explanation:
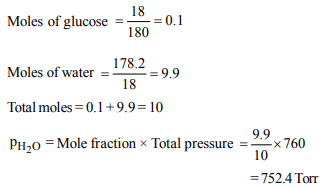
10.A mixture of ethyl alcohol and propyl alcohol has a vapour
pressure of 290 mm Hg at 300 K. The vapour pressure of
propyl alcohol is 200 mm Hg. If the mole fraction of ethyl
alcohol is 0.6, its vapour pressure (in mm Hg) at the same
temperature will be
a) 360
b) 350
c) 300
d) 700
Explanation: