1. Which of the following aqueous solution will have highest
depression in freezing point?
a) 0.1 M Urea
b) 0.1 M Sucrose
c) 0.1 M \[AlCl_{3}\]
d) 0.1 M \[K_{4}\left[Fe\left(CN\right)_{6}\right]\]
Explanation:

2. The depression in freezing point for 1 M urea, 1 M glucose
and 1 M NaCl are in the ratio
a) 1 : 2 : 3
b) 3 : 2 : 2
c) 1 : 1 : 2
d) None of these
Explanation: Urea and glucose are non electrolytes and 1 M NaCl =2M non electrolyte.
1:1: 2 (for NaCl i =2)
3.Which one of the following aqueous solutions will have the
lowest freezing point?
a) 0.1 molal solution of urea
b) 0.1 molal solution of sucrose
c) 0.1 molal solution of sodium chloride
d) 0.1 molal solution of calcium chloride
Explanation: 0.1 m CaCl2 . Concentration of particles = 0.3 m (Since i = 3)
4. The depression of freezing point is directly proportional to
a) mole fraction of the solution
b) molarity of the solution
c) molality of the solution
d) molarity of the solvent
Explanation:

5. A 0.5 molal solution of ethylene glycol in water is used as
coolant in a car. If the freezing point constant of water be
1.86°C per mole, the mixture shall freeze at
a) 0.93°C
b) –0.93°C
c) 1.86°C
d) –1.86°C
Explanation:
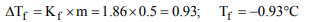
6. An aqueous solution freezes at –0.186°C \[\left(K_{f}=1.86,K_{b}=0.512\right)\] what is the elevation in boiling point?
a) 0.186
b) 0.512
c) 0.86
d) 0.0512
Explanation:
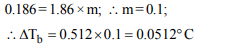
7. The molal freezing point constant for water is 1.86°C/m.
Therefore, the freezing point of 0.1 M NaCl solution in water
is expected to be
a) –1.86°C
b) –0.186°C
c) –0.372°C
d) +0.372°C
Explanation:
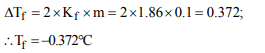
8. The molecular weight of benzoic acid in benzene as
determined by depression in freezing point method
corresponds to
a) ionization of benzoic acid
b) dimerization of benzoic acid
c) trimerization of benzoic acid
d) solvation of benzoic acid
Explanation: Benzoic acid forms a dimer in benzene.
9. The van't Hoff factor for 0.1 M \[Ba\left(NO_{3}\right)_{2}\] solution is 2.74.
The degree of dissociation is
a) 91.3%
b) 87%
c) 100%
d) 74%
Explanation:
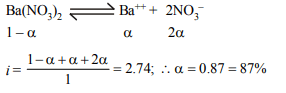
10. Formation of a solution from two components can be
considered as
(i) pure solvent \[\rightarrow \] separated solvent molecules, \[\triangle H_{1}\]
(ii) pure solute \[\rightarrow \] separated solute molecules, \[\triangle H_{2}\]
(iii) separated solvent and solute molecules \[\rightarrow\] solution, \[\triangle H_{3}\]
Solution so formed will be ideal if
a) \[\triangle H_{soln}=\triangle H_{1}+\triangle H_{2}-\triangle H_{3}\]
b) \[\triangle H_{soln}=\triangle H_{1}-\triangle H_{2}-\triangle H_{3}\]
c) \[\triangle H_{soln}=\triangle H_{3}-\triangle H_{1}-\triangle H_{2}\]
d) \[\triangle H_{soln}=\triangle H_{1}+\triangle H_{2}+\triangle H_{3}\]
Explanation: \[\triangle H_{soln}=\triangle H_{1}+\triangle H_{2}+\triangle H_{3}\]