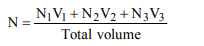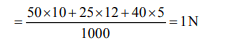1. If one mole of a substance is present in 1 kg of solvent, then
its concentration is called
a) Molar conc
b) Molal conc
c) Normality
d) Strength wt/wt
Explanation: If one mole of solute is present in 1 Kg of solvent the conc. is 1 m
2. Equal volumes of 0.1 M HCl and 0.1M NaOH are mixed. The
concentration of the resulting solution will be
a) 0.1 M
b) 0.05 M
c) 0.2 M
d) 0.0 M
Explanation: Solution will be neutral. Concentration of each will be 0.05 M since volume got doubled
3. When the solute is present in trace quantities the following
expression is used
a) gram per million
b) milligram percent
c) microgram percent
d) parts per million
Explanation: For very dil. solution the concentration is expressed in ppm
4. For preparing 0.1 N solution of a compound from its impure
sample of which the percentage purity is known, the weight
of the substance required will be
a) more than the theoretical weight
b) less than the theoretical weight
c) same as theoretical weight
d) None of the above
Explanation: Since the compound is impure more than theoretical weight is required.
5. What is the molarity of \[H_{2}SO_{4}\] solution that has a density
1.84 gm/cc at 35°C and contains 98% \[H_{2}SO_{4}\] by weight
a) 4.18 M
b) 8.14 M
c) 18.4 M
d) 18 M
Explanation:
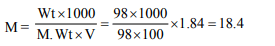
6. The amount of oxalic acid (mol. wt. 63) required to prepare
500 ml of its 0.10 N solution is
a) 0.315 g
b) 3.150 g
c) 6.300 g
d) 63.00 g
Explanation:
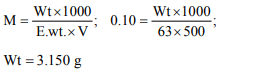
7. The molarity of the solution containing 7.1 g of \[Na_{2}SO_{4}\] in
100 ml of aqueous solution is
a) 2 M
b) 0.5 M
c) 1 M
d) 0.05 M
Explanation:
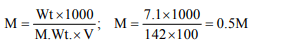
8. A solution of \[CaCl_{2}\] is 0.5 mol/litre, then the moles of chloride
ions in 500 ml. will be
a) 0.25
b) 0.50
c) 0.75
d) 1.00
Explanation:
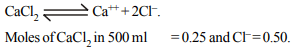
9. What will be the molality of a solution having 18 g of glucose
(mol. wt. = 180) dissolved in 500 g of water?
a)1 m
b) 0.5 m
c) 0.2 m
d) 2 M
Explanation:
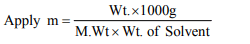
10. 50 ml of 10N \[H_{2}SO_{4}\] , 25 ml of 12 N HCl and 40 ml of 5 N HNO3
were mixed together and the volume of the mixture was made
1000 ml by adding water. The normality of the resulting
solution will be
a) 1 N
b) 2 N
c) 3 N
d) 4 N
Explanation: