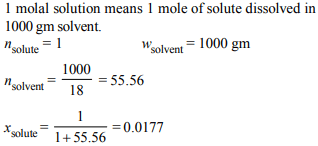1. If liquids A and B form an ideal solution
a) the enthalpy of mixing is zero
b) the entropy of mixing is zero
c) the free energy of mixing is zero
d) the free energy as well as the entropy of mixing are each
zero
Explanation:
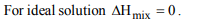
2. 25 ml of a solution of barium hydroxide on titration with a 0.1
molar solution of hydrochloric acid gave a titre value of 35
ml. The molarity of barium hydroxide solution was
a) 0.07
b) 0.14
c) 0.28
d) 0.35
Explanation:
3.During depression of freezing point in a solution the
following are in equilibrium
a) liquid solvent, solid solvent
b) liquid solvent, solid solute
c) liquid solute, solid solute
d) liquid solute, solid solvent
Explanation: Liquid solvent and solid solvent are in equilibrium.
4. A 0.0020 m aqueous solution of an ionic compound
\[Co\left(NH_{3}\right)_{5}\left(NO_{2}\right)Cl\] freezes at –0.00732 °C. Number of moles
of ions which 1 mol of ionic compound produces on being
dissolved in water will be \[\left(K_{f}=– 1.86°C/m\right)\]
a) 3
b) 4
c) 1
d) 2
Explanation:
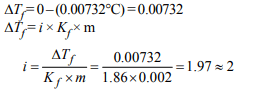
5. An aqueous solution is 1.00 molal in KI. Which change will
cause the vapour pressure of the solution to increase?
a) Addition of NaCl
b) Addition of \[ Na_{2}SO_{4}\]
c) Addition of 1.00 molal KI
d) Addition of water
Explanation: When the aqueous solution of one molal KI is diluted with water, concentration decreases, therefore the vapour pressure of the resulting solution increases
6. A solution of sucrose (molar mass = 342 g \[mol^{-1})\] has been
prepared by dissolving 68.5 g of sucrose in 1000 g of water.
The freezing point of the solution obtained will be ( \[K_{f}\] for water=1.86 K kg \[mol^{-1})\]
a) – 0.372°C
b) – 0.520°C
c) +0.372°C
d) – 0.570°C
Explanation:
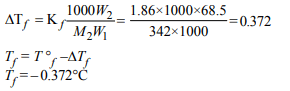
7. 25.3 g of sodium carbonate, \[Na_{2}CO_{3}\] is dissolved in enough
water to make 250 mL of solution. If sodium carbonate
dissociates completely, molar concentration of sodium ions,
\[Na^{+}\] and carbonate ions, \[CO_3^{2-}\] are respectively (molar mass of \[Na_{2}CO_{3}\] = 106 g \[mol ^{-1})\]
a) 0.955 M and 1.910 M
b) 1.910 M and 0.955 M
c) 1.90 M and 1.910 M
d) 0.477 M and 0.477 M
Explanation:
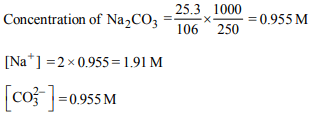
8. The freezing point depression constant for water is \[– 1.86ºC m^{-1}\] . If 5.00 g \[Na_{2}SO_{4}\] is dissolved in 45.0 g \[H_{2}O\] , the
freezing point is changed by – 3.82ºC. Calculate the van’t
Hoff factor for \[Na_{2}SO_{4}\]
a) 2.05
b) 2.62
c) 3.11
d) 0.381
Explanation:
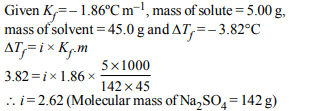
9. The van’t Hoff factor i for a compound which undergoes
dissociation in one solvent and association in other solvent
is respectively :
a) less than one and greater than one.
b) less than one and less than one
c) greater than one and less than one
d) greater than one and greater than one
Explanation: If compound dissociates in solvent i > 1 and on association i < 1
10. Mole fraction of the solute in a 1.00 molal aqueous solution is
a) 0.1770
b) 0.0177
c) 0.0344
d) 1.7700
Explanation: