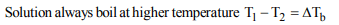1. The relationship between osmotic pressure at 273 K when
10g glucose \[\left(P_{1}\right)\] , 10 g urea \[\left(P_{2}\right)\] , and 10g sucrose \[\left(P_{3}\right)\] are
dissolved in 250 ml of water is
a) \[P_{1}>P_{2}>P_{3}\]
b) \[P_{3}>P_{2}>P_{1}\]
c) \[P_{2}>P_{1}>P_{3}\]
d) \[P_{2}>P_{3}>P_{1}\]
Explanation:
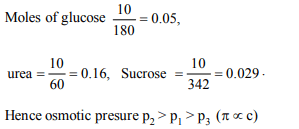
2. 0.1 M NaCl and 0.1 M CH3COOH are kept in separate
containers. If their osmotic pressures are \[P_{1}\] and \[P_{2}\]
respectively then what is the correct statement?
a) \[P_{1} > P_{2}\]
b) \[P_{1} = P_{2}\]
c) \[P_{1} < P_{2}\]
d) \[P_{1} = P_{2}\] =0 atm
Explanation: NaCl is strong electrolyte and CH3COOH weak electrolyte.
p1 > p2(value of i will be more in case of NaCl)
3. What happen when isotonic solution of A (mol. wt. 342) and
B (mol. wt. 60) are put into communication through
semipermeable membrane?
a) Transference of solvent from solution of A to that of B
takes place
b) Transference of solvent from solution of B to that of A
takes place
c) No transference of solvent from solution of A to that of
B takes place
d)Change in temperature of the solutions takes place
Explanation: Since solutions are isotonic, hence no transference of solvents
4. Which among the following will show maximum osmotic
pressure?
a) 1 M NaCl
b) 1 M \[MgCl_{2}\]
c) 1 M \[\left(NH_{4}\right)_{3}PO_{4}\]
d) 1 M \[Na_{2}SO_{4}\]
Explanation:
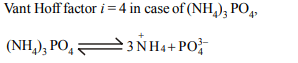
5. Isotonic solutions have
a) same boiling point
b) same vapour pressure
c) same melting point
d) same osmotic pressure
Explanation:

6. An aqueous solution of sucrose, \[C_{12}H_{22}O_{11}\] , containing 34.2
g/ litre has an osmotic pressure of 2.38 atmospheres at 17°C.
For an aqueous solution of glucose, \[C_{6}H_{12}O_{6}\] to be isotonic
with this solution, it would have
a) 34.2 g/lit of glucose
b) 17.1 g/lit of glucose
c) 18.0 g/lit of glucose
d) 36.0 g/lit of glucose
Explanation:

7. The osmotic pressure of 5% (mass-volume) solution of cane
sugar at 150°C (mol. mass of sugar = 342) is
a) 4 atm
b) 5.07 atm
c) 3.55 atm
d) 2.45 atm
Explanation:
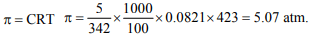
8. A 1% aqueous solution (mass by volume) of a certain
substance is isotonic with a 3% solution of dextrose i.e.
glucose (molar mass 180) at a given temperature. The molar
mass of the substance is
a) 60
b) 120
c) 180
d) 360
Explanation:
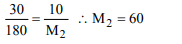
9. A 5% solution of cane sugar (mol. wt. = 342) is isotonic with
1% solution of substance X. The molecular weight of X is
a) 34.2
b) 171.2
c) 68.4
d) 136.8
Explanation:
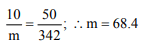
10. If the solution boils at a temperature \[T_{1}\] and the solvent at a
temperature \[T_{2}\] the elevation of boiling point is given by
a) \[T_{1}+T_{2}\]
b) \[T_{1}-T_{2}\]
c) \[T_{1}\]
d) \[T_{2}-T_{1}\]
Explanation: