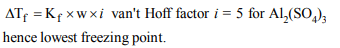1. Which of the following will have highest boiling point at 1
atm pressure?
a) 0.1 M NaCl
b) 0.1 M Sucrose
c) \[0.1 M BaCl_{2}\]
d) 0.1 M Glucose
Explanation: Conc. of particles will be highest in BaCl2 (Van't Hoff factor i = 3).
2. A solution of 1 molal concentration of a solute will have
maximum boiling point elevation when the solvent is
a) ethyl alcohol
b) acetone
c) benzene
d) chloroform
Explanation:

3. The normal boiling point of water is 373 K (at 760 mm Hg).
Vapour pressure of water at 298 K is 23 mm Hg. If enthalpy of
vaporisation is 40.656 \[kJ mol^{-1}\] , the boiling point of water at
23 mm Hg pressure will be
a) 250 K
b) 294.4 K
c) 51.6 K
d) 12.5 K
Explanation:

4.If the elevation in boiling point of a solution of 10 gm of
solute (mol. wt. = 100) in 100 gm of water is \[\triangle T_{b}\] , the
ebullioscopic constant of water is
a) 10
b) 10 \[\triangle T_{b}\]
c) \[\triangle T_{b}\]
d) \[\frac{\triangle T_{b}}{10}\]
Explanation:
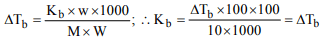
5. The rise in the boiling point of a solution containing 1.8 g of
glucose in 100 g of solvent is 0.1°C. The molal elevation
constant of the liquid is
a) 0.01 K/m
b) 0.1 K/m
c) 1 K/m
d) 10 K/m
Explanation:
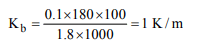
6.The boiling point of a solution of 0.11 g of a substance in 15
g of ether was found to be 0.1°C higher than that of pure
ether. The molecular weight of the substance will be \[(K_{b}\] = 2.16°K kg \[mol^{-1})\]
a) 148
b) 158
c) 168
d) 178
Explanation:
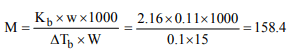
7. The boiling point of 0.1 molal \[K_{4}\left[ Fe \left(CN\right)_{6} \right]\] solution will be
(Given \[K_{b}\] for water = 0.52°K kg \[mol^{-1})\]
a) 100.52°C
b) 100.104°C
c) 100.26°C
d) 102.6°C
Explanation:
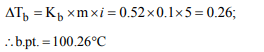
8. The freezing point of equimolal aqueous solution will be
highest for
a) \[C_{6}H_{5}NH_3^+Cl^{-}\]
b) \[Ca\left(NO_{3}\right)_{2}\]
c) \[La\left(NO_{3}\right)_{2}\]
d) \[C_{6}H_{12}O_{6}\]
Explanation: Glucose is non electrolyte hence depression in freezing point will be minimum, hence freezing point will be highest
9. If all the following four compounds were sold at the same
price, which would be cheapest for preparing an antifreeze
solution for a car radiator?
a) \[CH_{3}OH\]
b) \[C_{2}H_{5}OH\]
c) \[C_{2}H_{4}\left(OH\right)_{2}\]
d) \[C_{3}H_{5}\left(OH\right)_{3}\]
Explanation:
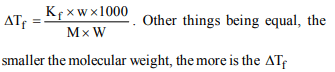
10. Which of the following 0.10 m aqueous solution will have
the lowest freezing point?
a) \[Al_{2}\left(SO_{4}\right)_{3}\]
b) KI
c) \[C_{6}H_{10}O_{5}\]
d) \[C_{12}H_{22}O_{11}\]
Explanation: