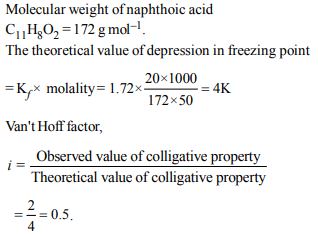
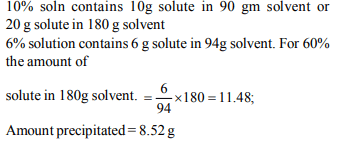1. When 20 g of naphthoic acid \[\left(C_{11}H_{8}O_{2}\right)\] is dissolved in 50 g
of benzene \[(K_{f}\] =1.72 K kg \[mol^{-1})\] , a freezing point depression
of 2 K is observed. The Van't Hoff factor (i) is
a) 0.5
b) 1
c) 2
d) 3
Explanation:
2. The Henry’s law constant for the solubility of \[N_{2}\] gas in water
at 298 K is \[1.0 × 10^{5}\] atm. The mole fraction of \[N_{2}\] in air is 0.8.
The number of moles of \[N_{2}\] from air dissolved in
10 moles of water at 298 K and 5 atm pressure is
a) \[4.0× 10^{-4}\]
b) \[4.0× 10^{-5}\]
c) \[5.0× 10^{-4}\]
d) \[4.0× 10^{-6}\]
Explanation:
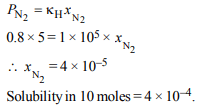
3. Dissolving 120 g of urea (mol. wt. 60) in 1000 g of water gave
a solution of density 1.15 g/mL. The molarity of the solution
is
a) 1.78 M
b) 2.00 M
c) 2.05 M
d) 2.22 M
Explanation:
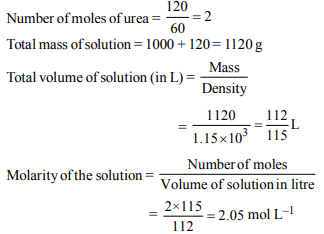
4.The freezing point (in °C) of a solution containing 0.1 g
of \[K_{3}\left[ Fe\left(CN\right)_{6}\right]\] (Mol. wt. 329) in 100 g of water \[(K_{f}\] =1.86 K kg \[mol^{–1})\] is
a) \[–2.3 × 10^{-2}\]
b) \[–5.7 × 10^{-2}\]
c) \[–5.7 × 10^{-3}\]
d) \[–1.2 × 10^{-2}\]
Explanation:
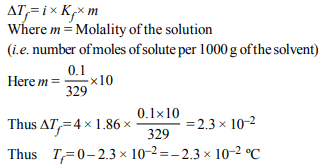
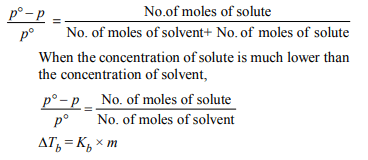
5. For a dilute solution containing 2.5 g of a non-volatile nonelectrolyte
solute in 100 g of water, the elevation
in boiling point at 1 atm pressure is 2°C. Assuming
concentration of solute is much lower than the concentration
of solvent, the vapour pressure (mm of Hg) of the solution is (take \[K_{b}\] =0.76 K kg \[mol^{-1})\]
a) 724
b) 740
c) 736
d) 718
Explanation:
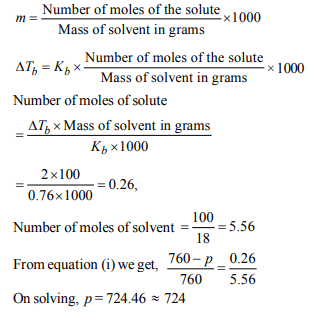
6. An X molal solution in carbon tetrachloride show the mole
fraction of solute equal to 0.23527 . The value of X is
a) 1.55
b) 1.82
c) 2.00
d) 2.16
Explanation:
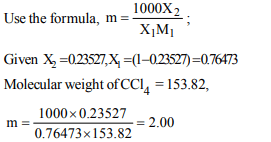
7. 3.0 molal sodium hydroxide solution has a density of
1.110 gm \[L^{-1}\] . The molarity of this solution is
a) 2.97
b) 3.05
c) 3.65
d) 4.11
Explanation:
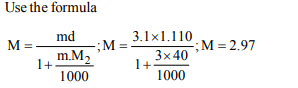
8. For a solution of two liquids A and B it was proved that
\[P_{S}=X_{A}\left(P^{\circ}_A-P^{\circ}_B\right)+P^{\circ}_B\] . The resulting solution will be
a) Non -ideal
b) ideal
c) semi-ideal
d) None of these
Explanation:
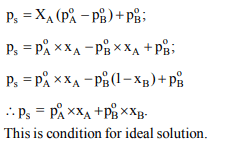
9. The vapour pressure of water at 50°C is 92.5 torr. What will
be the vapour pressure of solution which consists of 1 mole
of nonvolatile solute in 100 g of water at 50°C
a) 906 .5 torr
b) 94.2 torr
c) 91.8 torr
d) 90.8 torr
Explanation:
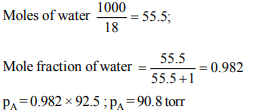
10. When 200g of 10% solution was cooled part of the solute
precipitated and the concentration of solution become 6% .
The mass of the precipitated solute is
a) 6.2 g
b) 8.5 g
c) 12.6 g
d) 14.0 g
Explanation: