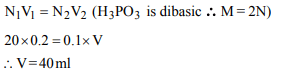1. A 0.1 molal aqueous solution of a weak acid is 30% ionized. If
\[K_{f}\] for water is 1.86°C/m, the freezing point of the solution will
be :
a) – 0.18°C
b) – 0.54°C
c) – 0.36°C
d) – 0.24°C
Explanation:
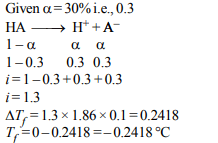
2. 200 mL of an aqueous solution of a protein contains its 1.26 g.
The osmotic pressure of this solution at 300 K is found to be
\[2.57 × 10^{-3}\] bar. The molar mass of protein will be (R = 0.083 L bar \[mol^{-1} K^{-1})\]
a) 51022 g \[mol^{-1}\]
b) 122044 g \[mol^{-1}\]
c) 31011 g \[mol^{-1}\]
d) 61038 g \[mol^{-1}\]
Explanation:
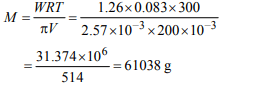
3. \[P_{A}\] and \[P_{B} \] are the vapour pressure of pure liquid components,
A and B, respectively of an ideal binary solution. If \[X_{A} \]
represents the mole fraction of component A, the total pressure
of the solution will be.
a) \[P_{A} +X_{A}\left(P_{B}-P_{A}\right)\]
b) \[P_{A} +X_{A}\left(P_{A}-P_{B}\right)\]
c) \[P_{B} +X_{A}\left(P_{B}-P_{A}\right)\]
d) \[P_{B} +X_{A}\left(P_{A}-P_{B}\right)\]
Explanation:
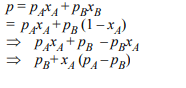
4. Freezing point of an aqueous solution is
(–0.186)°C. Elevation of boiling point of the same solution is
\[K_{b} = 0.512°C, K_{f}= 1.86°C,\] find the increase in boiling point.
a) 0.186°C
b) 0.0512°C
c) 0.092°C
d) 0.2372°C
Explanation:
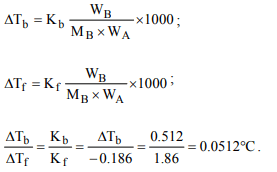
5. In mixture A and B components show -ve deviation as
a) \[\triangle V_{mix} >0\]
b) \[\triangle H_{mix} <0\]
c) A – B interaction is weaker than A – A and B – B interaction
d) A – B interaction is stronger than A – A and B – B
interaction
Explanation:
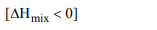
6. A pressure cooker reduces cooking time for food because
a) boiling point of water involved in cooking is increased
b) the higher pressure inside the cooker crushes the food
material
c) cooking involves chemical changes helped by a rise in
temperature
d) heat is more evenly distributed in the cooking space
Explanation: On increasing pressure, the temperature is also increased. Thus in pressure cooker due to increase in pressure the b.p. of water increases
7. In a 0.2 molal aqueous solution of a weak acid HX the degree
of ionization is 0.3. Taking kf for water as 1.85, the freezing
point of the solution will be nearest to
a) – 0.360º C
b) – 0.260º C
c) + 0.480º C
d) – 0.480º C
Explanation:
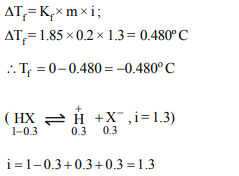
8. Which one of the following aqueous solutions will exihibit
highest boiling point ?
a) 0.015 M urea
b) 0.01 M \[KNO_{3} \]
c) 0.01 M \[Na_{2}SO_{4} \]
d) 0.015 M glucose
Explanation:
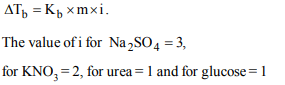
9. \[6.02 × 10^{20}\] molecules of urea are present in 100 ml of its
solution. The concentration of urea solution is
a) 0.02 M
b) 0.01 M
c) 0.001 M
d) 0.1 M
Explanation:
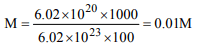
10. To neutralise completely 20 mL of 0.1 M aqueous solution of
phosphorous acid \[\left(H_{3}PO_{3} \right)\] , the value of 0.1 M aqueous KOH
solution required is
a) 40 mL
b) 20 mL
c) 10 mL
d) 60 mL
Explanation: