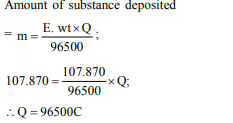1. The standard electrode potentials of four elements A, B, C
and D are –3.05, –1.66, –0.40 and +0.80. The highest
chemical reactivity will be exhibited by :
a) A
b) B
c) C
d) D
Explanation: 'A'
2. The aqueous solution of which of the following
decomposes on passing electric current
a) cane sugar
b) urea
c) methanol
d) potassium iodide
Explanation: potassium iodide
3. The standard electrode potential of the half cells are given
below
\[Zn^{2+}+2e^-\rightarrow Zn; E = – 7.62 V\]
\[Fe^{2+}+2e^-\rightarrow Fe; E = – 7.81 V\]
The emf of the cell, \[Fe^{2+}+Zn\rightarrow Zn ^{2+}+ Fe\] is
a) 1.54 V
b) -1.54 V
c) – 0.19 V
d) + 0.19 V
Explanation: – 0.19 V
4.Two electrolytic cells, one containing acidified ferrous
chloride and another acidified ferric chloride, are connected
in series. The ratio of iron deposited at cathodes in the two
cells will be :
a) 3 : 1
b) 2 : 1
c) 1 : 1
d) 3 : 2
Explanation: 3 : 2
5. On heating one end of a piece of a metal, the other end
becomes hot because of
a) resistance of the metal
b) mobility of atoms in the metal
c) energised electrons moving to the other end
d) minor perturbation in the energy of atoms
Explanation: On heating one end of a piece of a metal, the other end becomes hot because of energised electrons moving to the other end
6. 4.5 g of aluminium (at. mass 27 amu) is deposited at
cathode from \[Al^{3+}\] solution by a certain quantity of
electric charge. The volume of hydrogen produced at
STP from \[H^{+}\] ions in solution by the same quantity of
electric charge will be
a) 44.8 L
b) 22.4 L
c) 11.2 L
d) 5.6 L
Explanation: 5.6 L
7. On passing 0.5 Faraday of electricity through molten sodium
chloride, sodium deposited at cathode will be :
a) 29.25 g
b) 11.50 g
c) 58.50 g
d) 0.00 g
Explanation: 11.50 g
8. Which of the following is the use of electrolysis?
a) Electrorefining
b) Electroplating
c) Both (a) and (b)
d) Neither (a) nor (b)
Explanation:
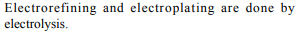
9.Which of the following will form the cathode with respect to
iron anode in an electrolytic cell?
a) Mg
b) AI
c) Cu
d) Zn
Explanation:
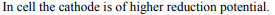
10. The number of coulombs required for the deposition of
107.870 g of silver is
a) 96500
b) 48250
c) 193000
d) 10000
Explanation: