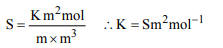1.Standard electrode potential for Sn4+ / Sn2+ couple is + 0.15 V
and that for the \[Cr^{3+}/Cr\] couple is – 0.74 V. These two couples
in their standard state are connected to make a cell. The cell
potential will be :
a) + 1.19 V
b) + 0.89 V
c) + 0.18 V
d) + 1.83 V
Explanation:
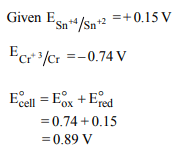
2. If the E°cell for a given reaction has a negative value, then
which of the following gives the correct relationships for the
values of \[\triangle G^{\circ}\] and \[K_{eq}\] ?
a) \[\triangle G^{\circ}>0: K_{eq} >1\]
b) \[\triangle G^{\circ}< 0: K_{eq} >1\]
c) \[\triangle G^{\circ}<0: K_{eq} < 1\]
d) \[\triangle G^{\circ}>0: K_{eq} <1\]
Explanation:
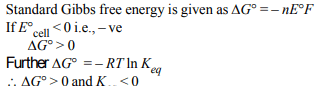
3. A solution contains \[Fe^{2+},Fe^{3+}\] and \[I^{-}\] ions. This solution
was treated with iodine at 35°C. E° for \[Fe^{3+}/Fe^{2+}\] is + 0.77 V
and E° for \[I_{2}/2I^{–} = 0.536 V.\] The favourable redox reaction is :
a) \[I_{2}\] will be reduced to \[I^{-}\]
b) There will be no redox reaction
c) \[I^{-}\] will be oxidised to \[I_{2}\]
d) \[Fe^{2+}\] will be oxidised to \[Fe^{3+}\]
Explanation:
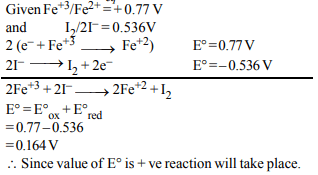
4. Limiting molar conductivity of \[NH_{4}OH\]
\[ i.e.,\wedge^{\circ}_{m\left(NH_{4}OH\right)}\] is equal to :
a) \[\wedge^{\circ}_{m\left(NH_{4}Cl\right)}+\wedge^{\circ}_{m\left(NaCl\right)}-\wedge^{\circ}_{m\left(NaOH\right)}\]
b) \[\wedge^{\circ}_{m\left(NaOH\right)}+\wedge^{\circ}_{m\left(NaCl\right)}-\wedge^{\circ}_{m\left(NH_{4}Cl\right)}\]
c) \[\wedge^{\circ}_{m\left(NH_{4}OH\right)}+\wedge^{\circ}_{m\left(NH_{4}Cl\right)}-\wedge^{\circ}_{m\left(HCl\right)}\]
d) \[\wedge^{\circ}_{m\left(NH_{4}Cl\right)}+\wedge^{\circ}_{m\left(NaOH\right)}-\wedge^{\circ}_{m\left(NaCl\right)}\]
Explanation:
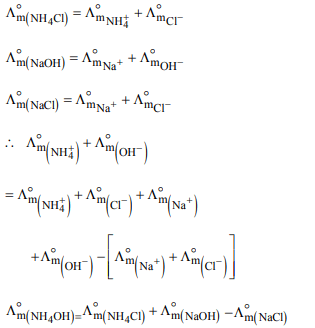
5. Standard reduction potentials of the half reactions are given
below :
\[F_{2}\left(g\right)+2e^{-}\rightarrow 2F^{-}\left(aq\right);E° = + 2.85 V\]
\[Cl_{2}\left(g\right)+2e^{-}\rightarrow 2Cl^{-}\left(aq\right);E° = + 1.36 V\]
\[Br_{2}\left(l\right)+2e^{-}\rightarrow 2Br^{-}\left(aq\right);E° = + 1.06 V\]
\[I_{2}\left(s\right)+2e^{-}\rightarrow 2I^{-}\left(aq\right);E° = + 0.53 V\]
The strongest oxidising and reducing agents respectively are :
a) \[F_{2}\] and \[I^{-}\]
b) \[Br_{2}\] and \[Cl^{-}\]
c) \[Cl_{2}\] and \[Br^{-}\]
d) \[Cl_{2}\] and \[I_{2}\]
Explanation:

6. Molar conductivities ( \[\wedge^°_m\] ) at infinite dilution of NaCl, HCl
and \[CH_{3}COONa\] are 126.4, 425.9 and 91.0 S \[cm^{2}mol^{-1}\] respectively. \[\wedge^°_m\] for CH3COOH will be :
a) 425.5 S \[cm^{2}mol^{-1}\]
b) 180.5 S \[cm^{2}mol^{-1}\]
c) 290.8 S \[cm^{2}mol^{-1}\]
d) 390.5 S \[cm^{2}mol^{-1}\]
Explanation:
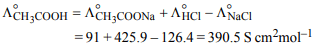
7. A hydrogen gas electrode is made by dipping platinum
wire in a solution of HCl of pH = 10 and by passing hydrogen
gas around the platinum wire at one atm pressure. The
oxidation potential of electrode would be ?
a) 0.59 V
b) 0.118 V
c) 1.18 V
d) 0.059 V
Explanation:
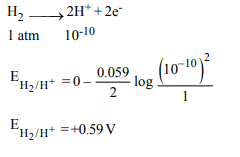
8. At 25°C molar conductance of 0.1 molar aqueous solution
of ammonium hydroxide is 9.54 \[ohm^{-1} cm^{2}mol^{-1}\] and at
infinite dilution its molar conductance is 238 \[ohm^{-1} cm^{2}mol^{-1}\] .
The degree or ionisation of ammonium hydroxide at the
same concentration and temperature is :
a) 20.800%
b) 4.008%
c) 40.800%
d) 2.080%
Explanation:
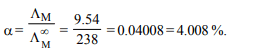
9. A button cell used in watches functions as following
\[Zn(s) + Ag_{2}O(s) + H_{2}O(l) \rightleftharpoons 2Ag(s) + Zn^{2+}(aq) + 2OH^{-}(aq)\]
if half cell potentials are:
\[Zn^{2+}\left(aq\right)+2e^{-}\rightarrow Zn\left(s\right);E^{\circ} = – 0.76 V\]
\[Ag_{2}O\left(s\right)+H_{2}O(l) +2e^{-}\rightarrow 2Ag\left(s\right)+2OH^{-}\left(aq\right);E^{\circ} = 0.34 V\]
The cell potential will be :
a) 0.42 V
b) 0.84 V
c) 1.34 V
d) 1.10 V
Explanation:

10. Conductivity (unit Siemen’s S) is directly proportional to
area of the vessel and the concentration of the solution in it
and is inversely proportional to the length of the vessel
then the unit of the constant of proportionality is
a) S m \[mol^{-1}\]
b) \[Sm^{2} mol^{-1}\]
c) \[S^{-2}m^{2} mol\]
d) \[S^{2}m^{2} mol^{-2}\]
Explanation: