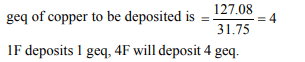1.An electrolytic cell contains a solution of \[Ag_{2}SO_{4}\] and has
platinum electrodes. A current is passed until 1.6 gm of \[O_{2}\]
has been liberated at anode. The amount of silver deposited
at cathode would be
a) 107.88 gm
b) 1.6 gm
c) 0.8 gm
d) 21.60 gm
Explanation:
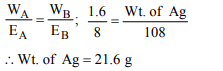
2. 1.08 g of pure silver was converted into silver nitrate and its
solution was taken in a beaker. It was electrolysed using
platinum cathode and silver anode. 0.01 Faraday of electricity
was passed using 0.15 volt above the decomposition
potential of silver. The silver content of the beaker after the
above shall be
a) 0 g
b) 0.108 g
c) 1.08 g
d) None of these
Explanation:
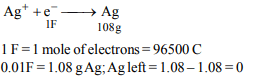
3. A current of 2.0 A passed for 5 hours through a molten metal
salt deposits 22.2 g of metal (At wt. = 177). The oxidation
state of the metal in the metal salt is
a) +1
b) +2
c) +3
d) +4
Explanation:
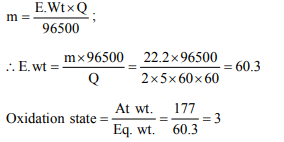
4. A 5 ampere current is passed through a solution of zinc
sulphate for 40 minutes. Find the amount of zinc deposited
at the cathode
a) 40.65 g
b) 4.065 g
c) 0.4065 g
d) 65.04 g
Explanation:
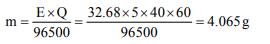
5. On passing a current of 1.0 ampere for 16 min and 5 sec
through one litre solution of \[ CuCl_{2}\] , all copper of the solution
was deposited at cathode. The strength of \[ CuCl_{2}\] solution
was (Molar mass of Cu = 63.5, Faraday constant = \[96500 C mol^{-1}\] ).
a) 0.07 M
b) 0.2 N
c) 0.005 M
d) 0.02 N
Explanation:
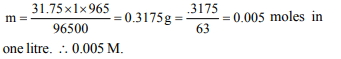
6. In a solution of \[ CuSO_{4}\] how much time will be required to
precipitate 2 g copper by 0.5 ampere current ?
a) 12157.48 sec
b) 102 sec
c) 510 sec
d) 642 sec
Explanation:
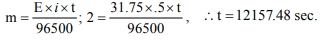
7. What is the amount of chlorine evolved when 2 amperes of
current is passed for 30 minutes in an aqueous solution of
NaCl?
a) 66 g
b) 1.32 g
c) 33 g
d) 99 g
Explanation:
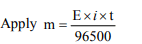
8. When 9.65 coulombs of electricity is passed through a
solution of silver nitrate (atomic mass of Ag = 108 g \[mol^{-1})\] , the amount of silver deposited is
a) 16.2 mg
b) 21.2 mg
c) 10.8 mg
d) 6.4 mg
Explanation:

9. The charge required to deposit 9 g of Al from \[Al^{3+}\] solution
is (At. wt. of Al = 27.0)
a) 3216.3 C
b) 96500 C
c) 9650 C
d) 32163 C
Explanation:
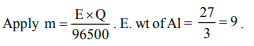
10. The quantity of electricity needed to deposit 127.08 g of
copper is
a) 1 Faraday
b) 4 Coulombs
c) 4 Faraday
d) 1 Ampere
Explanation: