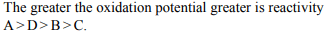1. If salt bridge is removed from two half-cells the voltage
a) drops to zero
b) does not change
c) increases gradually
d) increases rapidly
Explanation:

2. In a salt bridge KCl is used because
a) it is an electrolyte
b) it is good conductor of electricity
c) the transport number of \[K^{+}\] and \[Cl^{-}\] ions are nearly same
or both have same ionic mobility
d) it is ionic compound
Explanation: In a salt bridge KCl is used because the transport number of \[K^{+}\] and \[Cl^{-}\] ions are nearly same or both have same ionic mobility
3. The reference electrode is made by using
a) \[ZnCl_{2}\]
b) \[CuSO_{4}\]
c) \[HgCl_{2}\]
d) \[Hg_{2}Cl_{2}\]
Explanation:
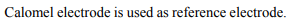
4. The standard hydrogen electrode potential is zero, because
a) there is no potential difference between the electrode
and the solution
b) hydrogen ions acquire electrons from a platinum
electrode
c) it has been measured accurately
d) it has been defined that way
Explanation:
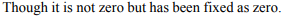
5. The standard reduction potentials E° for the half reactions
are as
\[Zn\rightarrow Zn^{2+}+2e^{-};E^{\circ}=0.76V\]
\[Fe\rightarrow Fe^{2+}+2e^{-};E^{\circ}=0.41V\]
The EMF for the cell reaction will be
a) –0.3 V
b) 0.35 V
c) 1.17 V
d) –1.17 V
Explanation:
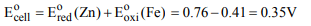
6. The standard electrode potential (E°) for \[OCl^{-}/Cl^{-}\] and
\[Cl^{-}/\frac{1}{2}Cl_{2}\] respectively are 0.94 V and –1.36 V. The E° value
for \[OCl^{-}/\frac{1}{2}Cl_{2}\] will be
a) –0.42 V
b) –2.20 V
c) 0.52 V
d) 1.04 V
Explanation:
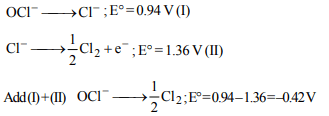
7. The standard reduction potential for \[Fe^{2+}/Fe\] and
\[Sn^{2+}/Sn\] electrodes are –0.44 and –0.14 volt respectively.
For the cell reaction
\[Fe^{2+}+Sn\rightarrow Fe+Sn^{2+}\]
the standard emf will be
a) +0.30 V
b) –0.58 V
c) +0.58 V
d) –0.30 V
Explanation:
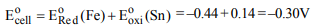
8. The emf of the cell
\[Ni/Ni^{2+}\left(1.0M\right)\parallel Au^{3+}\left(1.0M\right)/ Au \]
is \[[E^{\circ}\] for \[Ni^{2+}/Ni=-0.25 V;\] \[E^{\circ}\] for \[Au^{3+}/Au= 1.5 V]\]
a) +1.25 V
b) +1.75 V
c) -1.25 V
d) -1.75 V
Explanation:
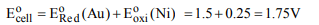
9. An unknown metal M displaces nickel from nickel (II)
sulphate solution but does not displace manganese from
manganese sulphate solution. Which order represents the
correct order of reducing power?
a) Mn > Ni > M
b) Ni > Mn > M
c) Mn > M > Ni
d) M > Ni > Mn
Explanation:
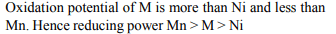
10. Electrode potentials \[\left(E^{\circ} _{red}\right)\] of four elements A, B, C, D are
–1.36, –0.32, 0, –1.26 V respectively. The decreasing reactivity
order of these elements is
a) A, D, B and C
b) C, B, D and A
c) B, D, C and A
d) C, A, D and B
Explanation: