1. Given \[ E^{\circ}_{Cr^{3+}/Cr}= –0.72 V,E^{\circ}_{Fe^{2+}/Fe}= – 0.42 V\]
The
potential for the cell
\[Cr\mid Cr^{3+}\left(0.1M\right)\parallel Fe^{2+}\left(0.01M\right)\mid Fe\]
is
a) 0.26 V
b) 0.336 V
c) – 0.339
d) 0.26 V
Explanation:
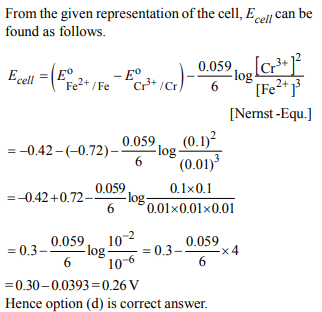
2. In a fuel cell methanol is used as fuel and oxygen gas is used
as an oxidizer. The reaction is
\[CH_{3}OH\left(l\right)+3/2O_{2}\left(g\right)\rightarrow CO_{2}\left(g\right)+2H_{2}O\left(l\right)\]
At 298 K standard Gibb’s energies of formation for \[ CH_{3}OH\left(l\right) ,H_{2}O\left(l\right)\] and \[CO_{2}\left(g\right)\] are –166.2 ,–237.2 and – 394.4 kJ \[mol^{-1}\]
respectively. If standard enthalpy of combustion of methonal
is – 726 kJ \[mol^{-1}\] , efficiency of the fuel cell will be:
a) 87%
b) 90%
c) 97%
d) 80%
Explanation:
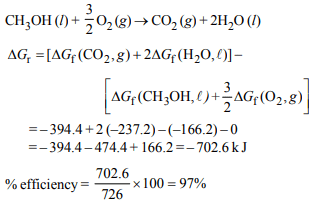
3.Given:
\[E^{\circ}_{Fe^{3+}/Fe}= –0.036V\]
\[E^{\circ}_{Fe^{2+}/Fe}= –0.439V\]
The value of standard electrode potential for the change,
\[Fe^{3+}\left(aq\right)+e^{-}\rightarrow Fe^{2+}\left(aq\right)\]
will be:
a) 0.385 V
b) 0.770 V
c) –0.270 V
d) –0.072 V
Explanation:

4. The Gibbs energy for the decomposition of \[Al_{2}O_{3}\] at 500°C is
as follows :
\[\frac{2}{3}Al_{2}O_{3}\rightarrow \frac{4}{3}Al+O_{2},\triangle _{r}G=+966 kJ mol^{-1}\]
The potential difference needed for electrolytic reduction of
\[Al_{2}O_{3}\] at 500°C is at least
a) 4.5 V
b) 3.0 V
c) 2.5 V
d) 5.0 V
Explanation:
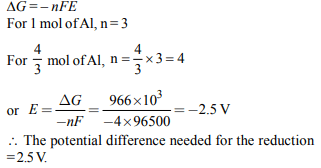
5.The correct order of \[E^{\circ}_{M^{2+}/M}\] values with negative sign for
the four successive elements Cr, Mn, Fe and Co is
a) Mn > Cr > Fe > Co
b) Cr < Fe > Mn > Co
c) Fe > Mn > Cr > Co
d) Cr > Mn > Fe > Co
Explanation:
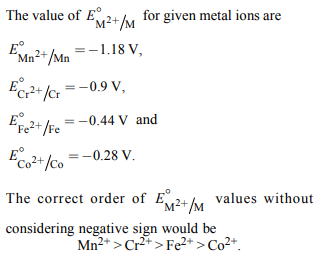
6. The reduction potential of hydrogen half-cell will be negative
if :
a) \[p\left(H_{2}\right)\] = 1 atm and \[\left[H^{+}\right] = 2.0 M\]
b) \[p\left(H_{2}\right)\] = 1 atm and \[\left[H^{+}\right] = 1.0 M\]
c) \[p\left(H_{2}\right)\] = 2 atm and \[\left[H^{+}\right] = 1.0 M\]
d) \[p\left(H_{2}\right)\] = 2 atm and \[\left[H^{+}\right] = 2.0 M\]
Explanation:
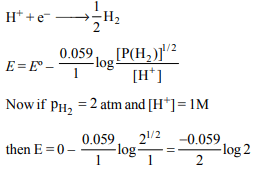
7. Consider the following cell reaction:
\[2Fe\left(s\right)+O_{2}\left(g\right)+4H^{+}\left(aq\right)\rightarrow 2Fe^{2+}\left(aq\right)+2H_{2}O\left(l\right);E^{\circ}=1.67V\]
At \[\left[Fe^{2+}\right]=10^{-3}M, p\left(O_{2}\right)\] = 0.1 atm and pH = 3, the cell
potential at 25ºC is
a) 1.47 V
b) 1.77 V
c) 1.87 V
d) 1.57 V
Explanation:
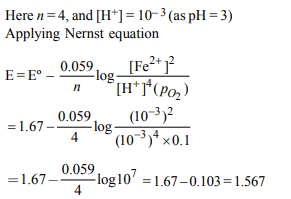
8. Resistance of 0.2 M solution of an electrolyte is 50 Ω . The
specific conductance of the solution is 1.3 S \[m^{-1}\] . If resistance
of the 0.4 M solution of the same electrolyte is 260 Ω , its molar
conductivity is :
a) 6.25 × \[10^{-4}\] S \[m^{2} mol^{-1}\]
b) 625 × \[10^{-4}\] S \[m^{2} mol^{-1}\]
c) 62.5 S \[m^{2} mol^{-1}\]
d) 6250 S \[m^{2} mol^{-1}\]
Explanation:
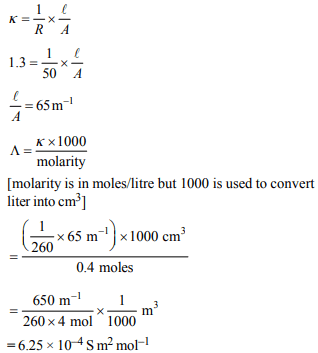
9. The standard reduction potentials for Zn2+/Zn,
\[Ni^{2+}/Ni\] and \[Fe^{2+}/Fe\] are –0.76,–0.23 and –0.44 V respectively.
The reaction \[X+Y^{2+}\rightarrow X^{2+}+Y\] will be spontaneous
when
a) X = Ni, Y = Fe
b) X = Ni, Y = Zn
c) X= Fe, Y = Zn
d) X= Zn, Y = Ni
Explanation:
10. Given : \[E^{\circ}_{Cr^{3+}/Cr}=-0.74 V;E^{\circ}_{MnO_4^-/Mn^{2+}}=1.51 V\]
\[E^{\circ}_{Cr_{2}O_7^{2-}/Cr^{3+}}=1.33 V;E^{\circ}_{Cl/Cl^{-}}=1.36 V\]
Based on the data given above, strongest oxidising agent
will be :
a) Cl
b) \[Mn^{2+}\]
c) \[Cr^{3+}\]
d) \[MnO_4^-\]
Explanation:
