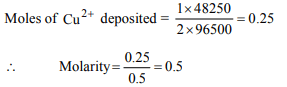1. A 1.0 M with respect to each of the metal halides \[AX_{3},BX_{2},CX_{3}\] and \[DX_{2}\] is electrolysed using platinum electrodes. If
\[E^{\circ}_{A^{3+}/A}=1.50 V,E^{\circ}_{B^{2}/B}=0.3 V,E^{\circ}_{C^{3+}/C}=-0.74 V,E^{\circ}_{D^{2}/D}=-2.37 V\]
The correct sequence in which the
various metals are deposited at the cathode is
a) A, B, C, D
b) A, B, C
c) D, C, B, A
d) C, B, A
Explanation:

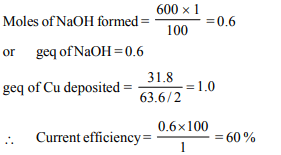
2. Electrolysis of NaCl solution with inert electrodes for certain
period of time gave \[600 cm^{3}\] of 1.0 M NaOH in the electrolytic
cell. During the same period 31.80 g of copper was deposited
in a copper voltmeter in series with the electrolytic cell. What
is the percent current efficiency in the electrolytic cell ? (At.
wt. of Cu = 63. 6)
a) 40
b) 25
c) 60
d) 50
Explanation:
3. Absolute ionic mobility is the speed of ion under the electric
field of and its dimension
a) 5 V across a distance of 5 cm, \[m^{2}V^{-1}S^{-1}\]
b) 10 V across a distance of 5 cm, \[mV^{-1}S^{-1}\]
c) 5 V across a distance of 10 cm, \[m^{2}V^{-2}S^{-1}\]
d) None of these
Explanation:
4. Given the cell reactions
\[MX \left(s\right) +e^{-}\rightarrow M \left(s\right) +X^{-} \left(aq\right), E^{\circ}=0.207V\]
and \[M^{+} \left(aq\right) +e^{-}\rightarrow M \left(s\right) , E^{\circ}=0.799 V\]
The solubility of MX (s) at 298 K is
a) \[1.0 \times 10^{-5} molL^{-1}\]
b) \[1.0 \times 10^{-4} molL^{-1}\]
c) \[1.0 \times 10^{-10} molL^{-1}\]
d) \[1.0 \times 10^{-9} molL^{-1}\]
Explanation:
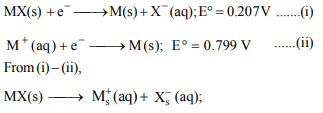
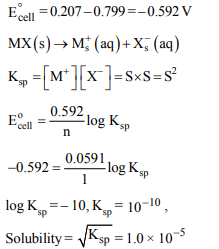
5.Electrolysis is carried out in three cells
(A) 1.0 M \[CuSO_{4}\] Pt electrode
(B) 1.0 M \[CuSO_{4}\] copper electrodes
(C) 1.0 M KCl Pt electrodes
If volume of electrolytic solution is maintained constant in
each of the cell, which is correct set of pH changes in (A), (B)
and (C) cell respectively ?
a) decrease in all the three
b) increase in all the three
c) decrease, constant, increase
d) increase, constant, increase
Explanation:
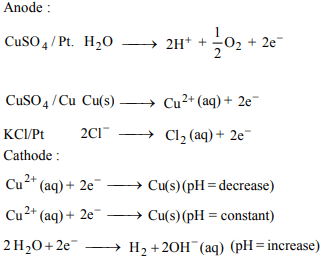
6.The reversible reduction potential of pure water is – 0.414 V
under 1 atm \[H_{2}\] pressure. If the reduction is considered to
be \[2H^{+}+2e \rightarrow H_{2}\] . Calculate the \[\left[H^{+}\right]\] of pure water
a) \[1.02\times10^{-7}\]
b) \[1.02\times10^{-9}\]
c) \[2.01\times10^{-7}\]
d) \[2.01\times10^{-9}\]
Explanation:
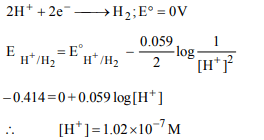
7. 1.0 L each of a buffer containing 1 mole \[NH_{3}\] and 1 mol of
\[NH_4^+\] were placed in the cathodic and anodic half-cells and
965 C of electricity was passed. If anodic and cathodic half
cells reactions involve oxidation and reduction of water only
as
\[2H_{2}O\rightarrow 4H^{+}+O_{2}-4e^{-};\]
\[2H_{2}O+2e\rightarrow H_{2}+2OH^{-}\]
Then pH of
a) cathodic solution will increase
b) anodic solution will decrease
c) both the solutions will remain practically constant
d) both the solutions will increase
Explanation:
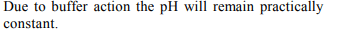
8. The pH of 0.5 L of 1.0 M NaCl after the electrolysis for 965
seconds using 5.0 A current (100% efficiency) is
a) 1.0
b) 13.0
c) 12.7
d) 1.3
Explanation:
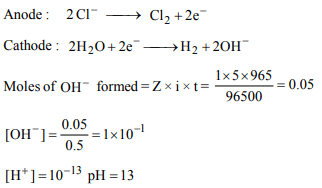
9. The emf of a particular voltaic cell with the cell reaction
\[Hg_2^{2+} +H_{2}\rightleftharpoons 2Hg + 2H^{+}\]
is 0.65 V. The maximum electrical work of this cell when 0.5 g
of \[H_{2}\] is consumed.
a) \[– 3.12 × 10^{4} J\]
b) \[–1.25 × 10^{5} J\]
c) \[25.0 × 10^{6} J\]
d) None
Explanation:
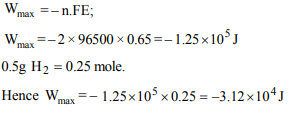
10. 48250 C of electricity was required to deposit all the copper
present in 0.5 L of CuSO4 solution using inert electrodes.
The molarity of solution was (Assume volume constant)
a) 0.50 M
b) 2.50 M
c) 0.25 M
d) 1.00 M
Explanation: