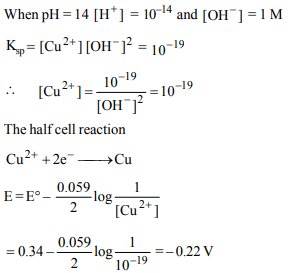1. The unit of ionic mobility is
a) \[m^{–2}V^{–1}s^{–1}\]
b) \[m^{2}V^{–1}s^{–1}\]
c) \[m^{–2}Vs^{–1}\]
d) \[m^{2}V^{–2}s^{–1}\]
Explanation:
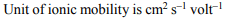
2. If \[\rho\] is the resistance in ohm of a centimeter cube, generally
called the specific resistance of the substance constituting
the conductor, the resistance r of the layer containing ''a''
cubes is given by
a) \[\frac{1}{r}=\frac{1}{\rho}+\frac{1}{\rho}+.....\]
b) \[\frac{1}{r}=\frac{1}{\rho a}+\frac{1}{\rho a}+.....\]
c) \[r=a/\rho\]
d) \[r=\rho+\rho+....\]
Explanation:

3. The mathematical expression for law of independent
migration of ions and Ostwald’s dilution law are given by
a) \[\wedge=\wedge^{\circ}_{m}-BC^{1/2}\]
b) \[\wedge^{\circ}=F\left(U_{+}+U_{-}\right)\]
c) \[\wedge^{\circ}_{m}=V_{+}\lambda_{+}+V_{-}\lambda_{-}\]
d) \[\frac{\wedge^{\circ}}{\wedge_{m}}=\frac{1}{\wedge^{\circ}_{m}}+\frac{\wedge_{m}C}{K_{a}\left(\wedge^{\circ}_{m}\right)^{2}}\]
Explanation:
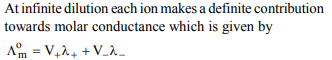
4. The ionic mobility of ions at infinite dilution is related to
ionic conductance by
a) \[\wedge^{\circ}=Fk\]
b) \[\wedge^{\circ}F=U_{+}+U_{-}\]
c) \[\wedge^{\circ}=U_{+}+U_{-}\]
d) \[\wedge^{\circ}=F \left(U_{+}+U_{-}\right)\]
Explanation:
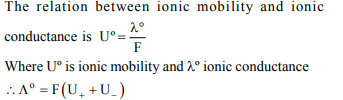
5.The value of molar conductance of HCl is greater than that
of NaCl at a given temperature and concentration because
a) ionic moblility of HCl is greater than that of NaCl
b) the dipole moment of NaCl is greater than that of HCl
c) NaCl is more ionic than HCl
d) HCl is Bronsted acid and NaCl is a salt of a strong acid
and strong base
Explanation:

6. Consider the following reactions
(i) \[Cd^{2+}\left(aq\right)+2e^{-}\rightarrow Cd\left(s\right),E^{\circ}=– 0.40 V\]
(ii) \[Ag^{2+}\left(aq\right)+e^{-}\rightarrow Ag\left(s\right),E^{\circ}=0.80 V\]
For the galvanic cell involving the above reactions. Which
of the following is not correct ?
a) Molar concentration of the cation in the cathodic
compartment changes faster than that of the cation in
the anodic compartment
b) \[E_{cell}\] increase when \[Cd^{2+}\] solution is diluted
c) Twice as many electrons pass through the cadmium
electrode as through silver electrode
d) \[E_{cell}\] decreases when \[Ag^{+}\] solution is diluted
Explanation:
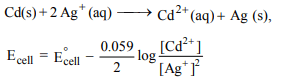
7. Conductance of 0.1 M KCl (conductivity = X \[Ohm^{-1}cm^{-1})\]
filled in a conductivity cell is Y \[Ohm^{-1}\] . If the conductance
of 0.1 M NaOH filled in the same cell is Z \[Ohm^{-1}\] , the molar
conductance of NaOH will be
a) \[ 10^{3}\frac{XZ}{Y}\]
b) \[ 10^{4}\frac{XZ}{Y}\]
c) \[ 10\frac{XZ}{Y}\]
d) \[ 0.1\frac{XZ}{Y}\]
Explanation:
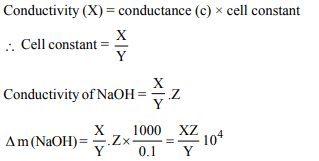
8. In electrolytic reduction of a nitroarene with 50% current
efficiency 20.50 g of the compound is reduced by 2 × 96500 C
of electric charge. The molar mass of the compound is
a) 123.0 g
b) 61.5 g
c) 10.2 g
d) 20.5 g
Explanation:
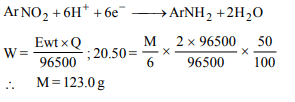
9. On electrolysing \[K_{2}SO_{4}\] solution using inert electrodes,
1.68 L (STP) of gases was obtained. How many moles of \[MnO_4^-\]
could be reduced to \[ Mn^{2+}\] by the same quantity of
electricity ?
a) 0.02
b) 0.15
c) 0.20
d) 0.10
Explanation:
10.The standard reduction potential for \[ Cu^{2+}/Cu\] is + 0.34.
Calculate the reduction potential at pH = 14 for the above
couple. \[\left(K_{sp}Cu \left(OH\right)_{2}= 1 × 10^{-19}\right)\]
a) – 0.22 V
b) + 0.22 V
c) – 0.44 V
d) + 0.44 V
Explanation: