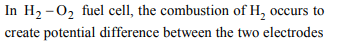1. EMF of a cell in terms of reduction potential of its left and
right electrodes is
a) \[E = E_{left} - E_{right}\]
b) \[E = E_{left} + E_{right}\]
c) \[E = E_{right} - E_{left}\]
d) \[E =-\left( E_{right} + E_{left}\right)\]
Explanation:

2. If \[\phi\] denotes reduction potential, then which is true?
a) \[E^{\circ}_{cell}=\phi_{right}-\phi_{left}\]
b) \[E^{\circ}_{cell}=\phi_{left}+\phi_{right}\]
c) \[E^{\circ}_{cell}=\phi_{left}-\phi_{right}\]
d) \[E^{\circ}_{cell}=-\left(\phi_{left}+\phi_{right}\right)\]
Explanation:

3.What will be the emf for the given cell
\[Pt\mid H_{2}\left(P_{1}\right)\mid H^{+}\left(aq\right)\mid \mid H_{2}\left(P_{2}\right)\mid Pt\]
a) \[\frac{RT}{f}log \frac{P_{1}}{P_{2}}\]
b) \[\frac{RT}{2f}log \frac{P_{1}}{P_{2}}\]
c) \[\frac{RT}{f}log \frac{P_{2}}{P_{1}}\]
d) None of these
Explanation:
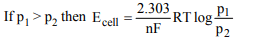
4. Which of the following reaction is possible at anode?
a) \[2 Cr^{3+}+7H_{2}O\rightarrow Cr_{2}O_7^{2-}+14 H^{+}\]
b) \[F_{2}\rightarrow 2F^{-}\]
c) \[\left(1/2\right)O_{2}+2H^{+}\rightarrow H_{2}O\]
d) None of these
Explanation:
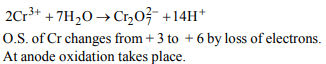
5. For a cell reaction involving a two-electron change, the
standard e.m.f. of the cell is found to be 0.295 V at 25ºC. The
equilibrium constant of the reaction at 25ºC will be
a) \[29.5 × 10^{-2}\]
b) 10
c) \[1 × 10^{10}\]
d) \[1 × 10^{-10}\]
Explanation:
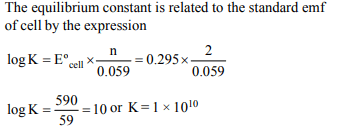
6. Standard reduction electrode potentials of three metals A, B
& C are respectively + 0.5 V, – 3.0 V & –1.2 V. The reducing
powers of these metals are
a) A > B > C
b) C > B > A
c) A > C > B
d) B > C > A
Explanation:
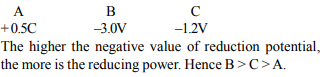
7. When during electrolysis of a solution of \[AgNO_{3}\] 9650
coulombs of charge pass through the electroplating bath,
the mass of silver deposited on the cathode will be
a) 10.8 g
b) 21.6 g
c) 108 g
d) 1.08 g
Explanation:
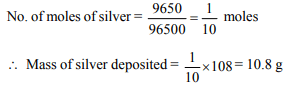
8. For the redox reaction :
\[Zn\left(s\right)+Cu^{2+}\left(0.1M\right)\rightarrow Zn^{2+}\left(1 M\right)+Cu\left(s\right)\]
taking place in a cell, \[E^{\circ}_{CeII}\] is 1.10 volt. \[E^{\circ}_{CeII}\] for the cell will
be \[2.303\frac{RT}{F}=0.0591\]
a) 1.80 volt
b) 1.07 volt
c) 0.82 volt
d) 2.14 volt
Explanation:
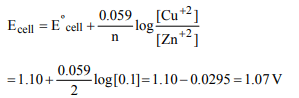
9. Several blocks of magnesium are fixed to the bottom of a
ship to
a) make the ship lighter
b) prevent action of water and salt
c) prevent puncturing by under-sea rocks
d) keep away the sharks
Explanation:

10. In a hydrogen-oxygen fuel cell, combustion of hydrogen
occurs to
a) produce high purity water
b) create potential difference between two electrodes
c) generte heat
d) remove adsorbed oxygen from electron surfaces
Explanation: