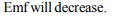1. Specific conductance of 0.1 M sodium chloride solution is \[1.06 × 10^{-2} ohm^{-1} cm^{-1}\] . Its molar conductance in \[ ohm^{-1} cm^{2}mol ^{-1}\] is
a) \[1.06 × 10^{2}\]
b) \[1.06 × 10^{3}\]
c) \[1.06 × 10^{4}\]
d) \[5.3 × 10^{2}\]
Explanation:
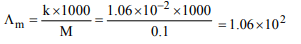
2. Molar conductivity of a solution is \[1.26 × 10^{2}\] Ω-1 \[cm^{2}mol ^{-1}\] .
Its molarity is 0.01. Its specific conductivity will be
a) \[1.26 × 10^{-5} \]
b) \[1.26 × 10^{-3} \]
c) \[1.26 × 10^{-4} \]
d) 0.0063
Explanation:
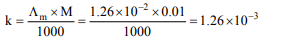
3. Molar ionic conductivities of a two-bivalent electrolytes
\[x^{2+}\] and \[y^{2-}\] are 57 and 73 respectively. The molar
conductivity of the solution formed by them will be
a) 130 S \[cm^{2}mol ^{-1}\]
b) 65 S \[cm^{2}mol ^{-1}\]
c) 260 S \[cm^{2}mol ^{-1}\]
d) 187 S \[cm^{2}mol ^{-1}\]
Explanation:
4. The equivalent conductivity of 0.1 M weak acid is 100 times
less than that at infinite dilution. The degree of dissociation
of weak acid will be
a) 100
b) 10
c) 0.01
d) 0.001
Explanation:
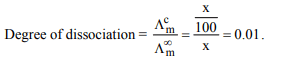
5.An electrochemical cell is set up as follows :
Pt \[\left(H_{2}, 1 atm\right)\] /0.1 M HCl/0.1 M acetic acid/\[\left(H_{2}, 1 atm\right)\] Pt
EMF of this cell will not be zero because
a) the temperature is constant
b) the pH of 0.1 M HCl and 0.1 M acetic acid is not the
same
c) acids used in the two compartments are different
d) EMF of a cell depends on molarities of the acids used
Explanation:

6. Which one of the following reaction occurs at the cathode?
a) \[2OH^{-}\rightarrow H_{2}O+O+2e^{-}\]
b) \[Ag\rightarrow Ag ^{+}+e^{-}\]
c) \[Fe^{2+}\rightarrow Fe ^{3+}+e^{-}\]
d) \[Cu^{2+}+2e^{-}\rightarrow Cu\]
Explanation:
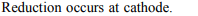
7. Which of the following statements is true for an
electrochemical cell?
a) Reduction occurs at \[H_{2}\] electrode
b) \[H_{2}\] is cathode and Cu is anode
c) \[H_{2}\] is anode and Cu is cathode
d) Oxidation occurs at Cu electrode
Explanation:
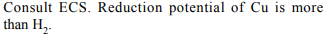
8. On the basis of the information available from the reaction
\[\frac{4}{3}Al+O_{2}\rightarrow \frac{2}{3}Al_{2}O_{3},\] \[\triangle G=-827 kJmol^{-1}\] Of \[O_{2}\]
the
minimum e.m.f. required to carry out electrolysis of \[Al_{2}O_{3}\] is (F = 96500 C \[mol^{-1} )\]
a) 4.28 V
b) 6.42 V
c) 8.56 V
d) 2.14 V
Explanation:
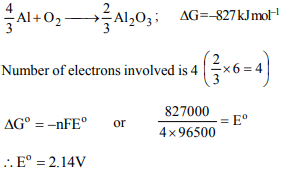
9. In the electrochemical reaction
\[2F e^{3+} +Zn\rightarrow Zn^{2+} +2F e^{2+} \]
on increasing the concentration of \[F e^{2+} \]
a) increases cell emf
b) increases the current flow
c) decreases the cell emf
d) alters the pH of the solution
Explanation:
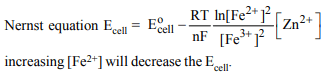
10. In the cell reaction
\[Cu \left(s\right)+2Ag^{+}\left(aq\right)\rightarrow Cu^{2+}\left(aq\right) +2Ag \left(s\right)\]
\[E^{\circ}_{cell}= 0.46 V.\] By doubling the concentration of Cu2+, \[E^{\circ}_{cell}\] will become
a) doubled
b) halved
c) increases but less than double
d) decreases by a small fraction
Explanation: