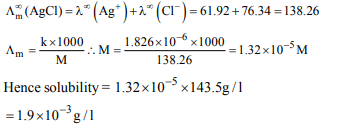1.Four successive members of the first row transition elements
are listed below with atomic numbers. Which one of them is
expected to have the highest \[ E^{\circ}_{M^{3+}/M^{2+}}\] value ?
a) Cr(Z = 24)
b) Mn(Z = 25)
c) Fe(Z = 26)
d) Co(Z = 27)
Explanation:
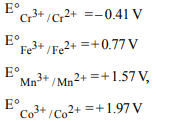
2. Electrolysis of dilute aqueous NaCl solution was carried out
by passing 10 milli ampere current. The time required to liberate
0.01 mol of \[H_{2}\] gas at the cathode is (1 Faraday = 96500C \[mol^{-1})\]
a) \[9.65 × 10^{4} sec\]
b) \[19.3× 10^{4} sec\]
c) \[28.95 × 10^{4} sec\]
d) \[38.6 × 10^{4} sec\]
Explanation:
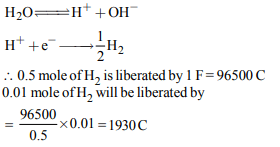
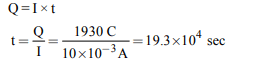
3. Which of the following solutions of KCl will have the highest
value of specific conductance?
a) 1.0 N
b) 0.1 N
c) \[1.0 ×10^{-2}N\]
d) \[1.0 ×10^{-3}N\]
Explanation:
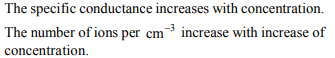
4. Which of the following statements is not correct?
a) The equivalent conductance of an electrolyte increases
on dilution
b) The equivalent conductance of an electrolyte decreases
on dilution
c) The degree of ionization of a weak electrolyte is given
by \[\alpha =\lambda_{c}/\lambda_{0}\] , where \[\lambda_{c}\] and \[\lambda_{0}\] are equivalent
conductances at concentration c and zero respectively
d) In case of a weak electrolyte on dilution, specific
conductance decreases but its equivalent conductance
increases
Explanation:
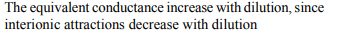
5. Which of the following statements is wrong ?
a) Electrolysis of an aqueous sodium hydroxide solution
liberates \[H_{2}\] gas at the cathode and \[O_{2}\] gas at the anode
b) Electrolysis of dil. \[H_{2}SO_{4}\] liberates \[H_{2}\left(g\right)\] at cathode
and \[O_{2}\left(g\right)\] at the anode
c) \[\triangle G^{\circ}=nFE^{\circ}\] for a spontaneous reaction
d) \[E=E^{\circ}-\frac{0.059}{n}logQ\] , Where Q = reaction quotient
Explanation:
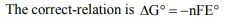
6. In the electrolysis of \[CuCl_{2}\] solution, the mass of the cathode
increased by 3.2g. What occured at the copper anode ?
a) 0.12 litre of \[Cl_{2}\] was liberated
b) 0.56 litre of \[O_{2}\] was liberated
c) 0.1 mol \[Cu^{2+}\] passed into the solution
d) 0.05 mol of \[Cu^{2+}\] passed into the solution
Explanation:
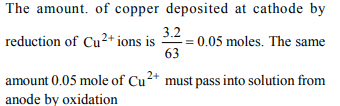
7. Copper can be deposited from acidified copper sulphate
and alkaline copper cyanide both. If the same current is
passed for the definite time, which of the following is correct?
a) The amount of copper deposited from acidic copper
sulphate will be higher
b) The amount of copper deposited from alkaline copper
cyanide will be more.
c) The same amount of Cu will be deposited
d) No Cu will be deposited
Explanation:
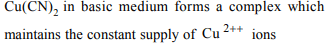
8. The EMF of the cell \[Tl/Tl^{+}\left(0.001 M\right)\parallel Cu^{2+}\left(0.01 M\right)/Cu\]
is
0.83. The cell EMF can be increased by
a) Increasing the concentration of \[Tl^{+}\] ions.
b) Increasing the concentration of \[Cu^{2+}\] ions.
c) Increasing the concentration of \[Tl^{+}\] and \[Cu^{2+}\] ions
d) None of these
Explanation:

9. What is the reaction taking place at the anode when an
aqueous solution of copper sulphate is electrolysed using
Pt–electrodes (inert) ?
a) \[ Cu^{2+}+ 2e^{-}\rightarrow Cu\]
b) \[ 2H^{+}+ 2e^{-}\rightarrow H_{2}\]
c) \[ 2H_{2}O\rightarrow O_{2}+ 4H^{+}+ 4e^{-}\]
d) \[ 2SO_4^{2-}\rightarrow S_{2}O_8^{2-}+ 2e^{-}\]
Explanation:
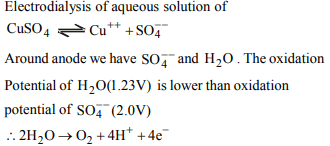
10. The specific conductance at 298 K of AgCl is
\[1.826 × 10^{-6} ohm^{-1}cm^{-1}\] . The ionic conductances of \[Ag^{+}\] and \[Cl^{-}\] are 61.92 and 76.34 respectively. What is the solubility
of AgCl in water ?
a) \[1.1\times 10^{-2}g^{-1}\]
b) \[1.9\times 10^{-3}g L^{-1}\]
c) \[2.1\times 10^{-5}g L^{-1}\]
d) \[2.1\times 10^{-6}g L^{-1}\]
Explanation: