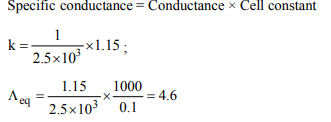1. Silver is monovalent and has atomic mass of 108. Copper is
divalent and has an atomic mass of 63.6. The same electric
current is passed for the same length of time through a
silver coulometer and a copper coulometer. If 27.0 g of silver
is deposited, then the corresponding amount of copper
deposited is
a) 63.60 g
b) 31.80 g
c) 15.90 g
d) 7.95 g
Explanation:
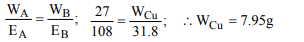
2. By passing 0.1 Faraday of electricity through fused sodium
chloride, the amount of chlorine liberated is
a) 35.45 g
b) 70.9 g
c) 3.545 g
d) 17.77 g
Explanation:
3.The unit of specific conductivity is
a) ohm \[cm^{-1}\]
b) ohm \[cm^{-2}\]
c) \[ohm^{-1}\] cm
d) \[ohm^{-1}\] \[cm^{-1}\]
Explanation:
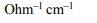
4. The cell constant of a given cell is \[0.47 cm^{-1}\] . The resistance
of a solution placed in this cell is measured to be 31.6 ohm.
The conductivity of the solution (in S cm–1 where S has
usual meaning) is
a) 0.15
b) 1.5
c) 0.015
d) 150
Explanation:
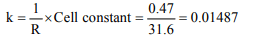
5. The specific conductivity of N/10 KCl solution at 20°C is
0.212 \[ohm^{-1} cm^{-1}\] and the resistance of the cell containing
this solution at 20°C is 55 ohm. The cell constant is
a) 4.616 \[cm^{-1}\]
b) 11.66 \[cm^{-1}\]
c) 2.173 \[cm^{-1}\]
d) 3.324 \[cm^{-1}\]
Explanation:
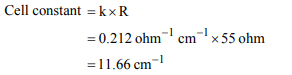
6. The resistance of 1 N solution of acetic acid is 250 ohm,
when measured in a cell of cell constant 1.15 \[cm^{-1}\] . The
equivalent conductance (in \[ohm^{-1} cm^{2}equiv^{-1})\] of 1 N acetic
acid will be
a) 4.6
b) 9.2
c) 18.4
d) 0.023
Explanation:
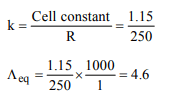
7. The equivalent conductance at infinite dilution of a weak
acid such as HF
a) can be determined by extrapolation of measurements of
dilute solutions of HCl, HBr and HI
b) can be determined by measurement of very dilute HF
solutions
c) can be determined from measurements of dilute
solutions of NaF, NaCl and HCl
d) is an undefined quantity
Explanation:
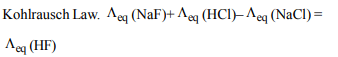
8. The unit of equivalent conductivity is
a) ohm cm
b) \[ ohm^{-1}\] \[cm^{+2}\] (g \[equivalent)^{-1}\]
c) ohm \[cm^{2}\] (g equivalent)
d) S \[cm^{-2}\]
Explanation:
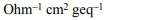
9. The conductivity of a saturated solution of \[BaSO_{4}\]
is \[ 3.06 × 10 ^{-6}ohm^{-1} cm^{-1}\] and its equivalent conductance is 1.53 \[ohm^{-1} cm^{2}equiv^{-1}\] . The \[K_{sp}\] for \[BaSO_{4}\] will be
a) \[4 × 10^{-12}\]
b) \[2.5 × 10^{-9}\]
c) \[2.5 × 10^{-13}\]
d) \[4 × 10^{-6}\]
Explanation:
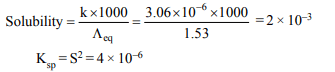
10. The resistance of 0.1 N solution of a salt is found to be \[2.5 × 10^{3}\] ohm . The equivalent conductance of the solution is
(cell constant = 1.15 \[cm^{-1})\]
a) 4.6
b) 5.6
c) 6.6
d) 7.6
Explanation: