1. Chlorine cannot displace
a) Fluorine from NaF
b) Iodine from NaI
c) Bromine from NaBr
d) None of these
Explanation:
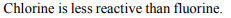
2. Standard potentials (E°) for some half-reactions are given
below :
(I) \[Sn ^{4+} +2e\rightarrow Sn ^{2+};E ^{\circ}= +0.15V\]
(II) \[2Hg ^{2+} +2e\rightarrow Hg_2^{2+};E ^{\circ}= 0.92 V .\]
(III) \[PbO_{2} +4H^{+}+2e\rightarrow Pb^{2+}+2H_{2}O;E ^{\circ}= +1.45V\]
based on the above, information which one of the following
statements is correct?
a) \[Sn^{4+}\] is a stronger oxidising agent than \[Pb^{4+}\]
b) \[Sn^{2+}\] is a stronger reducing agent than \[Hg_{2}^{2+}\]
c) \[Pb^{2+}\] is a stronger oxidising agent than \[Pb^{4+}\]
d) \[Pb^{2+}\] is a stronger reducing agent than \[Sn^{2+}\]
Explanation:

3. The oxidation potentails of A and B are +2.37 and +1.66 V
respectively. In chemical reactions
a) A will be replaced by B
b) A will replace B
c) A will not replace B
d) A and B will not replace each other
Explanation:
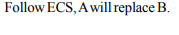
4. Electrode potential data are given below :
\[Fe^{+3}\left(aq\right)+e^{-}\rightarrow Fe^{+2}\left(aq\right);E° = +0.77 V\]
\[Al^{3+}\left(aq\right)+3e^{-}\rightarrow Al\left(s\right);E° = – 1.66 V\]
\[Br_{2}\left(aq\right)+2e^{-}\rightarrow 2Br^{-}\left(aq\right);E° = + 1.08V\]
Based on the data, the reducing power of \[Fe^{2+}\] , Al and \[Br^{-}\] will increase in the order
a) \[Br^{-}< Fe^{2+} < Al\]
b) \[ Fe^{2+}< Al < Br^{-}\]
c) \[ Al < Br^{-}< Fe^{2+}\]
d) \[ Al< Fe^{2+}< Br^{-}\]
Explanation:

5. Choose the correct statement from the following which is
related to the electrochemical series
a) Electrochemical series is not the arrangement of metals
and ions according to their reactivity
b) The metal ions at the top of the electrochemical series
are highly electronegative
c) Strongly electropositive metals can displace weakly
electropositive metals from their salt solution
d) All metals above hydrogen in the series do not displace
hydrogen from dilute acids
Explanation:

6. The standard reduction potentials of four elements are given
below. Which of the following will be the most suitable
reducing agent?
I = –3.04 V
II = – 1.90 V
III = 0 V
IV = 1.90 V
a) III
b) II
c) I
d) IV
Explanation:
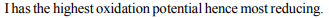
7. The standard reduction potentials at 298K for the following
half reactions are given against each
\[Zn^{2+}\left(aq\right)+2e\rightleftharpoons Zn\left(s\right); –0.762 V\]
\[Cr^{3+}\left(aq\right)+3e\rightleftharpoons Cr\left(s\right); –0.740 V\]
\[2H^{+}\left(aq\right)+2e\rightleftharpoons H_{2}\left(g\right); 0.00 V\]
\[Fe^{3+}\left(aq\right)+e\rightleftharpoons Fe^{2+}\left(aq\right);
0.770 V\]
Which is the strongest reducing agent?
a) \[Zn\left(s\right)\]
b) \[Cs\left(s\right)\]
c) \[H_{2}\left(g\right)\]
d) \[Fe^{3+}\left(aq\right)\]
Explanation:
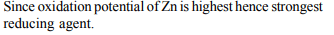
8. Zn gives H2 gas with \[H_{2}SO_{4}\] and HCl but not with \[HNO_{3}\]
because
a) Zn acts as oxidizing when reacts with \[HNO_{3}\]
b) \[HNO_{3}\] is weaker acid than \[H_{2}SO_{4}\] and HCl
c) In electrochemical series Zn is above hydrogen
d) \[NO_3^-\] is reduced in preference to hydronium
Explanation:

9. A smuggler could not carry gold by depositing iron on the
gold surface since
a) gold is denser
b) iron rusts
c) gold has higher reduction potential than iron
d) gold has lower reduction potential than iron
Explanation:
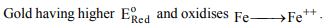
10. The metal that cannot displace hydrogen from dilute
hydrochloric acid is
a) aluminium
b) Iron
c) copper
d) zinc
Explanation:
