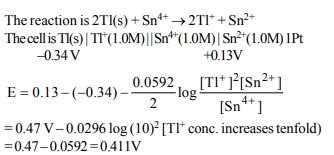1. The potential of a hydrogen electrode at pH=10 is
a) 0.59 V
b) zero volt
c) –0.59 V
d) 0.059 V
Explanation:
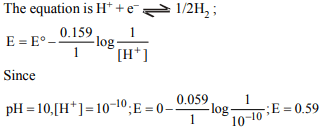
2. Equivalent conductance at infinite dilution, \[\lambda^{\circ}\] of \[NH_{4}Cl\] ,
NaOH and NaCl are 128.0, 217.8 and \[109.3 ohm^{-1} cm^{2} eq^{-1}\]
respectively. The equivalent conductance of 0.01 N \[NH_{4}OH\]
is \[9.30 ohm^{-1} cm^{2} eq^{-1}\] , then the degree of ionization of
\[NH_{4}OH\] at this temperature would be
a) 0.04
b) 0.1
c) 0.62
d) 0.39
Explanation:
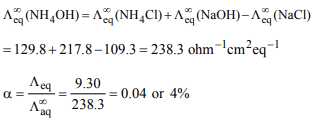
3. What is the standard cell potential E° for an electrochemical
cell in which the following reaction takes place
spontaneously ?
\[Cl_{2}\left(g\right)+2Br^{-}\rightarrow Br_{2}\left(aq\right)+2Cl^{-}\triangle G^{\circ}=-50.6KJ\]
a) 1.2 V
b) 0.53 V
c) 0.26 V
d) -0.53 V
Explanation:
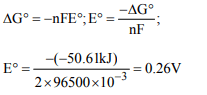
4. When electric current is passed through acidified water, 112
ml of hydrogen gas at STP collected at the cathode in 965
seconds. The current passed in amperes is
a) 1.0
b) 0.5
c) 0.1
d) 2.0
Explanation:

5. If the half cell reaction is \[A+e^{-}\rightarrow A^{-}\] has a large negative
reduction potential , it follows that
a) A is readily reduced
b) A is ready oxidised
c) \[A^{-}\] is readily reduced
d) \[A^{-}\] is readily oxidised
Explanation:
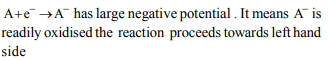
6. The electrochemical equivalent of silver is 0.001180 g. When
an electric current of 0.5 amp is passed through an aqueous
silver nitrate solution for 200 sec., the amount of silver
deposited is
a) 1.1180 g
b) 0.1180 g
c) 5.590 g
d) 0.5598 g
Explanation:
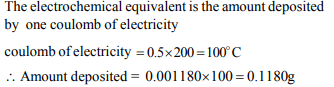
7. A gas X at 1 atm is bubbled through a solution containing a
mixture of 1 M \[Y^{-}\] and M \[Z^{-}\] at 25°C. If the reduction potential
of Z > Y > X, then,
a) Y will oxidize X and not Z
b) Y will oxidize Z and not X
c) Y will oxidize both X and Z
d) Y will reduce both X and Z
Explanation:

8. For the electrochemical cell, M|M+ || X– | X,
EoM+ /M= 0.44V and Eo (X/X–) = 0.33V.
From this data one can deduce that
a) \[M+X \rightarrow M ^{+}+X ^{-}\] is the spontaneous reaction
b) \[M ^{+}+X ^{-}\rightarrow M+X\] is the spontaneous reaction
c) \[E_{cell} = 0.77 V\]
d) \[E_{cell} = - 0.77 V\]
Explanation:
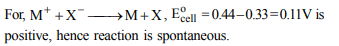
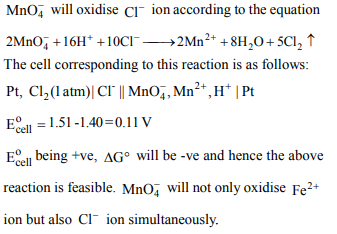
9. Standard electrode potential data are useful for understanding the suitability of an oxidant in a redox titration. Some half cell
reactions and their standard potentials are given below:
\[MnO_4^-\left(aq\right)+8H^{+}\left(aq\right)+5e^{-}\rightarrow Mn^{2+}\left(aq\right)+4H_{2}O\left(l\right);E^{\circ}=1.51 V\]
\[Cr_{2}O_7^{2-}\left(aq\right)+14H^{+}\left(aq\right)+6e^{-}\rightarrow 2Cr^{3+}\left(aq\right)+7H_{2}O\left(l\right);E^{\circ}=1.38 V\]
\[Fe^{3+}\left(aq\right)+e^{-}\rightarrow Fe^{2+}\left(aq\right);E^{\circ}=0.77 V\]
\[Cl_{2}\left(g\right)+2e^{-}\rightarrow 2Cl^{-}\left(aq\right);E^{\circ}=1.40 V\]
Identify the only incorrect statement regarding the quantitative estimation of aqueous Fe(NO3)2
a) \[MnO_4^-\] can be used in aqueous HCl
b) \[Cr_{2}O_7^{2-}\] can be used in aqueous HCl
c) \[MnO_4^-\] can be used in aqueous \[H_{2}SO_{4}\]
d) \[Cr_{2}O_7^{2-}\] can be used in aqueous \[H_{2}SO_{4}\]
Explanation:
10. A galvanic cell is constructed as follows. A half-cell consists
of a platinum wire immersed in a solution containing 1.0 M
of \[Sn^{2+}\] and 1.0 M of \[Sn^{4+}\] , and another half-cell has a thallium
rod immersed in a 1.0 M solution of \[Tl^{+}\] .
Given : \[Sn^{4+}\left(aq\right)+2e^{-}\rightarrow Sn^{2+}\left(aq\right);E^{\circ}=+0.13V\]
and \[Tl^{+}\left(aq\right)+e^{-}\rightarrow Tl\left(s\right);E^{\circ}=–0.34V\]
what is the cell voltage if the Tl+ concentration is increased
tenfold?
a) 0.411V
b) 4.101V
c) 0.492V
d) 0.222V
Explanation: