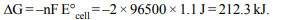1. Which reaction is not feasible?
a) \[2KI + Br_{2}\rightarrow2KBr + I_{2}\]
b) \[2KBr + I_{2}\rightarrow 2KI + Br_{2}\]
c) \[2KBr + Cl_{2}\rightarrow 2KCl + Br_{2}\]
d) \[2H_{2}O + 2F_{2}\rightarrow 4HF + O_{2}\]
Explanation:
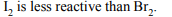
2. Which of the following will form a cell with the highest
voltage?
a) 1M \[Ag^{+},\] 1M \[Co^{2+}\]
b) 2M \[Ag^{+},\] 2M \[Co^{2+}\]
c) 0.1M \[Ag^{+},\] 2M \[Co^{2+}\]
d) 2M \[Ag^{+},\] 0.1M \[Co^{2+}\]
Explanation:
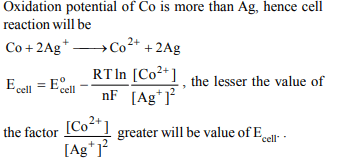
3. E° of a cell \[aA + bB\rightarrow cC+ dD \]
is
a) \[E + RT ln\frac{\left[a\right]^{A}\left[b\right]^{B}}{\left[c\right]^{C}\left[d\right]^{D}}\]
b) \[E + \frac{RT}{nF} ln\frac{\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}}\]
c) \[E + \frac{RT}{nF} ln\frac{\left[C\right]^{c}\left[d\right]^{D}}{\left[A\right]^{A}\left[B\right]^{B}}\]
d) \[E + \frac{RT}{nF} ln\frac{\left[a\right]^{A}\left[B\right]^{B}}{\left[C\right]^{C}\left[d\right]^{D}}\]
Explanation:
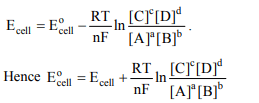
4. The standard EMF for the cell reaction,
\[Zn +Cu^{2+}\rightarrow Cu + Zn^{2+}\] is 1.1 volt at 25°C.
The EMF for the cell reaction, when 0.1 M \[Cu^{2+}\] and 0.1 M \[Zn^{2+}\] solutions are used, at 25°C is
a) 1.10 V
b) 0.10 V
c) -1.10 V
d) –0.110 V
Explanation:

5. What is the potential of half-cell consisting of
zinc electrode in 0.01 M \[ZnSO_{4}\] solution at 25°C \[\left(E^{\circ}_{OX}= 0.763 V\right)\]
a) 0.8221 V
b) 8.221 V
c) 0.5282 V
d) 9.282 V
Explanation:
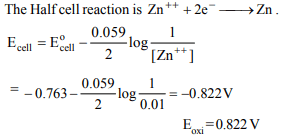
6. The oxidation potential of 0.05 M \[H_{2}SO_{4}\] is
a) –2 × 0.0591
b) –0.01 × 0.0591
c) –2.321 × 0.0591
d) +1 × 0.0591
Explanation:
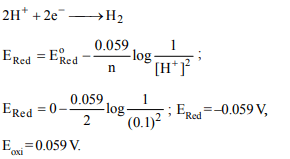
7. For the cell reaction,
\[Cu^{2+}\left[C_{1}\left(aq\right)\right]+Zn\left(s\right)\rightarrow Zn^{2+}\left(C_{2}\right)+Cu\left(s\right)\]
of an electrochemical cell, the change in free energy, \[\triangle\] G at a
given temperature is a function of
a) \[ln\left(C_{1}\right)\]
b) \[ln\left(\frac{C_{2}}{C_{1}}\right)\]
c) \[ln\left(C_{1}+C_{2}\right)\]
d) \[ln\left(C_{2}\right)\]
Explanation:
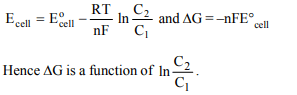
8. The relationship between standard reduction potential of a
cell and equilibrium constant is shown by
a) \[E^{\circ}_{Cell}=\frac{n}{0.059}log k_{C}\]
b) \[E^{\circ}_{Cell}=\frac{0.059}{n}log k_{C}\]
c) \[E^{\circ}_{Cell}=0.059 n log k_{C}\]
d) \[E^{\circ}_{Cell}=\frac{log k_{C}}{n}\]
Explanation:
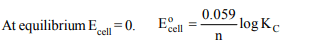
9. E° for the cell, \[Zn\mid Zn^{2+}\left(aq\right)\parallel Cu^{2+}\left(aq\right)\mid Cu\]
is 1.10 V at 25°C. The
equilibrium constant for the cell reaction
\[Zn+ Cu^{2+}\left(aq\right)\rightleftharpoons Cu +Zn^{2+}\left(aq\right)\]
is of the order of
a) \[10^{-37}\]
b) \[10^{37}\]
c) \[10^{-17}\]
d) \[10^{17}\]
Explanation:
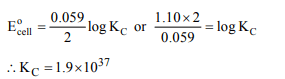
10. The standard EMF of Daniell cell is 1.10 volt. The maximum
electrical work obtained from the Daniell cell is
a) 212.3 kJ
b) 175.4 kJ
c) 106.15 kJ
d) 53.07 kJ
Explanation: