1. The emf of Daniell cell is 1.1 volt. If the value of Faraday is
96500 coulombs per mole, the change in free energy in kJ is
a) 212.30
b) –212.30
c) 106.15
d) -106.15
Explanation:
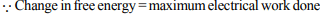
2. Pure water does not conduct electricity because it
a) has low boiling point
b) is almost unionised
c) is neutral
d) is readily decomposed
Explanation:
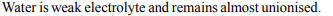
3. At cathode, the electrolysis of aqueous \[Na_{2}SO_{4}\] gives
a) Na
b) \[H_{2}\]
c) \[SO_{3}\]
d) \[SO_{2}\]
Explanation:
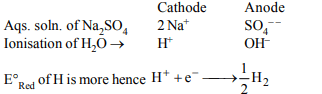
4. At anode in the electrolysis of fused NaCl
a) \[Na^{+}\] is oxidized
b) \[Cl^{-}\] is oxidized
c) Cl is reduced
d) Na is reduced
Explanation:

5. In electrolysis of NaCl when Pt electrode is taken then \[H_{2}\] is
liberated at cathode while with Hg cathode, it forms sodium
amalgam
a) Hg is more inert than Pt
b) More voltage is required to reduce \[H^{+}\] at Hg than at Pt
c) Na is dissolved in Hg while it does not dissolve in Pt
d) Conc. of \[H^{+}\] ions is larger when Pt electrode is taken
Explanation: More voltage is required to reduce \[H^{+}\] at Hg than at Pt
6. Which of the following reaction occurs at the cathode during
the charging of lead storage battery?
a) \[Pb^{2+}+2e^{-}\rightarrow Pb\]
b) \[Pb^{2+}+SO_4^{2-}\rightarrow PbSO_{4}\]
c) \[Pb \rightarrow Pb^{2+}+2e^{-}\]
d) \[PbSO_{4}+2H_{2}O\rightarrow 2PbO_{2}+4H^{+}+SO_4^{2-}+2e^{-}\]
Explanation: \[PbSO_{4}+2H_{2}O\rightarrow 2PbO_{2}+4H^{+}+SO_4^{2-}+2e^{-}\]
7.Reaction that takes place at graphite anode in dry cell is
a) \[Zn^{2+}+2e^{-}\rightarrow Zn\left(s\right)\]
b) \[Zn\left(s\right)\rightarrow Zn^{2+}+2e^{-}\]
c) \[Mn^{2+}+2e^{-}\rightarrow Mn\left(s\right)\]
d) \[Mn\left(s\right)\rightarrow Mn^{+}+e^{-}+1.5 V\]
Explanation: \[Zn\left(s\right)\rightarrow Zn^{2+}+2e^{-}\]
8.Which one of the following cells can convert chemical energy
of \[H_{2}\] and \[O_{2}\] directly into electrical energy?
a) Mercury cell
b) Daniell cell
c) Fuel cell
d) Lead storage cell
Explanation: Fuel cell
9. Hydrogen-Oxygen fuel cells are used in space craft to supply
a) power for heat and light
b) power for pressure
c) oxygen
d) water
Explanation:
10. As lead storage battery is charged
a) lead dioxide dissolves
b) sulphuric acid is regenerated
c) lead electrode becomes coated with lead sulphate
d) the concentration of sulphuric acid decreases
Explanation: H2SO4 regenerated