1. The \[pK_{a}\] of a weak acid, HA, is 4.80. The \[pK_{b}\] of a weak base,
BOH, is 4.78. The pH of an aqueous solution of the
corresponding salt, BA, will be
a) 9.58
b) 4.79
c) 9.22
d) 7.01
Explanation: 7.01
2. What will be the value of pH of 0.01 mol \[dm^{-3}CH_{3}COOH \left[K_{a}=1.74\times10^{-5}\right]\]
a) 3. 4
b) 3. 6
c) 3. 9
d) 3. 0
Explanation: 3. 4
3. The pH of a solution which is 0.1 M in HA and 0.5 M in NaA. \[ K_{a}\] for HA is \[1.8\times10^{-6}\]
a) 5.44
b) 6.44
c) 6.0
d) 4.73
Explanation: 6.44
4. Which of the following will produce a buffer solution when
mixed in equal volumes?
a) 0.1 mol \[dm^{-3}NH_{4}OH \] and 0.1 mol \[dm^{-3}HCl \]
b) 0.05 mol \[dm^{-3}NH_{4}OH \] and 0.1 mol \[dm^{-3}HCl \]
c) 0.1 mol \[dm^{-3}NH_{4}OH \] and 0.05 mol \[dm^{-3}HCl \]
d) 0.1 mol \[dm^{-3}CH_{4}COONa \] and 0.1 mol \[dm^{-3}NaOH \]
Explanation: 0.1 mol \[dm^{-3}NH_{4}OH \] and 0.05 mol \[dm^{-3}HCl \]
5.A solution which is \[10^{-3} \] M each in \[Mn^{2+}, Fe^{2+},Zn^{2+}\] and \[Hg^{2+}\] is treated with \[10^{-6} \] M sulphide ion. If \[K_{sp}\] of MnS, FeS, ZnS and HgS are \[10^{-15} \] , \[10^{-23} \] , \[10^{-20} \] and \[10^{-54} \] respectively which one will precipitate first
a) FeS
b) MgS
c) HgS
d) ZnS
Explanation: HgS
6. Which will not affect the degree of ionization?
a) Temperature
b) Concentration
c) Type of solvent
d) Current
Explanation: Current does not effect the degree of ionisation
7. At infinite dilution, the percentage ionisation for both strong
and weak electrolytes is
a) 1%
b) 20%
c) 50%
d) 100%
Explanation: Ionisation is 100% at infinite dilution
8.A 0.2 molar solution of formic acid is 3.2% ionized. Its
ionisation constant is
a) \[9.6 × 10^{-3}\]
b) \[2.1 × 10^{-4}\]
c) \[1.25 × 10^{-6}\]
d) \[4.8 × 10^{-5}\]
Explanation:
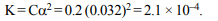
9. The hydrogen ion concentration of 0.2 N \[CH_{3}COOH\] which
is 40% dissociated is
a) 0.08 N
b) 0.12 N
c) 0.80 N
d) 1.2 N
Explanation:
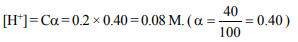
10. Degree of dissociation of 0.1 N CH3COOH is (Dissociation constant = \[1 × 10^{-5})\]
a) \[ 10^{-5}\]
b) \[ 10^{-4}\]
c) \[ 10^{-3}\]
d) \[ 10^{-2}\]
Explanation: \[ 10^{-2}\]